|
|
|
|
|
|
|
|

|
The Wet, Oxidizing Crust of Mars
Written by G. Jeffrey Taylor |
Herd and his co-workers find that as oxygen fugacity increases in a group of shergottites, 87Sr/86Sr and the ratio of lanthanum (La) to ytterbium (Yb) also increase, while 143Nd/144Nd decreases. They suggest these trends indicate that, compared to the Martian mantle, the crust is more oxidizing, has higher 87Sr/86Sr and La/Yb, and lower 143Nd/144Nd. Magmas formed in the mantle would have low oxygen fugacity. As magmas rose through the crust, they reacted with the surrounding rocks to varying extents, producing the observed chemical trends. How did the crust become more oxidizing than the mantle? They suggest that circulating hot water oxidized the crust. Alternatively, water-rich magmas might have crystallized in the crust, forming deposits of hydrous minerals. Subsequent magmas could react with the hydrated minerals to become more oxidizing. Whatever the details, the work by Herd and colleagues indicates that the mantle and crust differ significantly, that the crust has significant deposits of water, and many pristine magmas are modified by interaction with the crust.
Meteorites and the Crust and Mantle of Mars
Understanding how planets formed and how they evolved geologically requires knowing something about the composition of their interiors and surfaces. Martian meteorites give us an indirect, somewhat blurry glimpse of the Martian core, rocky mantle, and crust. Chris Herd and his colleagues are trying to pin down the oxidation conditions in the mantle and the crust by looking at chips of lava flows sent to us for free by impacts on Mars. The trick is to figure out which features of the rocks apply to the mantle and which apply to the crust.
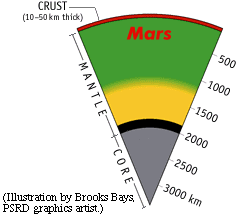 |
Data from Martian meteorites and orbiting spacecraft give hints about the nature of the interior of Mars. Chris Herd and his colleagues are trying to use Martian meteorites to determine the oxidation state of the mantle and crust of the red planet. |
Minerals Record the Oxidation State...
Iron typically occurs in two valence states, doubly-charged (Fe2+) and triply-charged (Fe3+). The amount of each depends largely on the oxidation conditions, hence on the oxygen fugacity. The concept of oxygen fugacity is completely foreign to most of us. Even the name is forbidding. But it is actually a fairly simple concept: Oxygen fugacity is just a measure of the amount of free or uncombined oxygen that is available in an environment. One confusing thing is that oxygen makes up about half the volume of virtually all magmas and rocks. That sounds pretty oxidizing, but most of that oxygen is chemically bound to silicon (which is usually the second most abundant element) and other positively-charged ions, and hence not freely available. Thus, only a little of it is available to alter the valence state (i.e., the charge) of iron in a magma or mineral. It is as if the oxygen were in an atmosphere that permeates a magma or rock. In fact, the fugacity is measured in terms of atmospheric pressure. This atmosphere is rather tenuous: in most magmas, oxygen fugacity ranges from 10-10 to 10-18 atmospheres of pressure. (10-10 means that the oxygen partial pressure is one ten-billionth of the pressure at the surface of Earth.)
With higher oxygen fugacity, there is more Fe3+ (ferric iron) and less Fe2+ (ferrous iron) in the iron-bearing minerals in a rock. In some cases the oxygen fugacity is so low that iron occurs as Fe2+ and uncharged (metallic) iron. Moon rocks are like that: they contain tiny bits of metallic iron.
Experiments and quantitative thermodynamic calculations allow us to determine the oxygen fugacity by measuring the amounts of ferrous and ferric iron in minerals, especially oxides. For example, one pair of minerals can range in composition from pure ulvospinel (Fe2TiO4, with all the Fe existing in the ferrous state) to magnetite (Fe3O4, with one-third of the iron being in the ferrous state and the rest ferric). Many experiments show that if both minerals are in equilibrium with each other, the amount of ferric iron in each is related to the oxygen fugacity.
 |
|
Electron microprobe image of iron-bearing minerals in Martian meteorite DaG 476 [Data link]. Brightness is proportional to the number of electrons bouncing off the mineral surfaces. This, in turn, is proportional to the average atomic weight of the material being bombarded with the electron beam, which is usually directly related to the percentage of iron atoms in that material. Ilm is ilmenite; TMt is titanomagnetite; Sf is iron sulfide. Dark surrounding material is composed of silicate minerals. |
...But Reading the Record is Tricky
In principle, all we need to do is to analyze the chemical composition, including the amounts of Fe2+ and Fe3+, in oxide minerals in a rock, plug the data into formulas that have been determined experimentally, and calculate the oxygen fugacity. Too bad things are not that simple. One complication is that the minerals occur in fine intergrowths. This means that they cannot be separated physically from the rock and analyzed by traditional chemical techniques that give the amounts of ferrous and ferric iron. Instead, we need to use an electron microprobe. This instrument is capable of analyzing tiny spots (only a micrometer or two across) on polished slices of a rock. This gets us away from the need to physically separate the minerals, but the electron probe analyzes elements only. It does not give us their valence state.
There are two ways to determine the amount of ferrous and ferric Fe from an electron microprobe analysis. One is to determine the elemental abundances very carefully and assume that the minerals have perfect, ideal chemical compositions. This allows us to divide the iron into Fe2+ and Fe3+ in just the right amounts for the composition of each mineral to be exactly correct. For example, ulvospinel has the ideal composition Fe2TiO4. The total amount of Fe and Ti are measured, and the amount of oxygen is adjusted until the ratio of Fe+Ti to O is exactly 3 to 4. The problem is that minerals are like people--they're not perfect. The ratio might be 2.9 to 4, or 3.1 to 4. This affects the calculated oxygen fugacity. The problem is also complicated by the presence of other elements, such as magnesium, chromium, aluminum, and manganese, in each mineral.
Another approach is to measure oxygen directly in the electron microprobe. This would seem to be pretty easy since there is so much oxygen. However, light elements like oxygen are notoriously difficult to analyze. For example, great care must be taken in selecting the correct oxygen peak in the x-ray spectrum produced in the electron microprobe. Furthermore, microprobe analyses must be corrected for assorted affects (such as x-ray absorption), and there are several choices available for oxygen. On top of all that, the analyses must be very precise because for modest amounts of ferric iron in an iron-bearing oxide the difference in total oxygen is quite small, less than 10% of the amount present.
Given all the uncertainties and experimental difficulties, Herd and his coworkers determined the Fe2+ and Fe3+ in both ways and calculated oxygen fugacity by two different methods. This painstaking work leads to some interesting correlations with other geochemical data and some intriguing interpretations about the Martian crust and mantle.
Oxidation State Correlated with Geochemical Parameters
Meenakshi Wadhwa measured the ratio of Europium (Eu) to gadolinium (Gd) in pyroxene crystals in Martian meteorites. Both are rare earth elements, and they behave in predictable ways during the formation and solidification of magma. Europium has the added virtue of occurring in two different oxidation states, as doubly-charged (Eu2+) and triply-charged (Eu3+). Gadolinium is less temperamental and remains as triply-charged Gd3+. The lucky thing is that Gd behaves almost exactly like Eu3+, so geochemists can figure out the amount of Eu in each valence state from the total amount of Eu and the amount of Gd. The ratio of doubly- to triply-charged Eu is proportional to the oxygen fugacity. So in principle, if you can figure out the ratio of doubly- to triply-charged europium, you can determine the oxidation conditions.
Herd and coworkers compared their calculated oxygen fugacities with Wadhwa's measured Eu and Gd concentrations in pyroxene in the same rocks. (The concentrations are expressed as the ratio of the concentration of each element in pyroxene to its concentration in the entire rock.) The result is a good positive correlation between Eu/Gd and oxygen fugacity.
 |
|
Eu/Gd in pyroxene (specifically the mineral augite) correlates with oxygen fugacity determined from mineral compositions. Because the values of oxygen fugacity are so tiny, they are usually expressed as a logarithm and often compared to some kind of standard conditions. For example, a useful comparison is the free oxygen associated with an assemblage of the minerals quartz (SiO2), fayalite (Fe2SiO4, and magnetite (Fe3O4), nicknamed the QFM buffer. So, on this diagram, an oxygen fugacity of -3 is 1000 times smaller than QFM. |
One concern with oxide minerals is that they continue to exchange oxygen after they have crystallized. This is a problem because we want to know the oxygen fugacity of the magma, not the rock after it formed. So, the correlation in the diagram above is important because the Eu and Gd are incorporated into pyroxene during crystallization. It indicates that the oxygen fugacity determined by Herd also applies to conditions during crystallization, hence in the magma.
Herd examined the relation between oxygen fugacity and other geochemical parameters. Like Meenakshi Wadhwa, he found that there is a good correlation between oxygen fugacity and 87Sr/86Sr, 143Nd/144Nd, and La/Yb (lanthanum/ytterbium). These parameters are all indicators of planetary crustal materials. As the crust formed, rubidium (a radioactive element that decays to 87Sr) is separated from strontium (Sr). This causes a continual increase in the 87Sr/86Sr ratio in the crust. Similarly, radioactive samarium-147 (147Sm) is separated from neodynmium (Nd). It tends to stay behind in the mantle, causing the mantle to increase in 143Nd/144Nd with time. La is also preferentially partitioned into the crust compared to Yb.
 |
 |
 |
|
These three figures show strong correlations between three geochemical parameters and oxygen fugacity. In each case, the more oxidizing conditions (higher oxygen fugacity, which increases to the right) are associated with isotopic or elemental ratios expected for the crust of Mars. Herd and coworkers suggest that this indicates reaction between reduced (low oxygen fugacity) mantle-derived magma and more oxidized crustal materials. |
What do these correlations mean? It might mean that the mantle of Mars is very heterogeneous in composition and oxidation state. The differences would all have to correlate with each other. Perhaps there are two distinct regions in the mantle and magmas from them mingle to differing extents, leading to the observed correlations. Alternatively, the two regions could represent the mantle and crust. In this case, magma formed in the mantle reacts chemically with the crust raising oxygen fugacity, 87Sr/86Sr, and La/Yb, and decreasing 143Nd/144Nd. This reaction happened to varying extent, accounting for the trends seen in the diagrams above.
Herd and his colleagues prefer the mantle-crust hypothesis and consider several materials that could cause the oxidation of primary, relatively reduced magmas. They focus on the type of material that was assimilated by ascending magmas. One type could be sedimentary materials rich in ferric iron, the substance that gives the red planet its color. However, assimilation of iron oxides alone cannot explain the variations in isotopic and elemental ratios, so other material must also be involved. Two likely candidates are oxidized lava flows (which could be buried to significant depths as volcanism constructed the crust), or pockets of hydrous minerals or rock-water mixtures. Both have the virtue of being able to explain the trends seen in the diagrams above. The figures below show two possible scenarios to explain the observed correlations between oxygen fugacity and geochemical parameters.
 |
|
Alternative scenarios for producing oxidized lava flows on Mars, assuming that the mantle is relatively reduced and uniform in composition. In the top figure (a), magma interacts with altered (weathered or chemically reacted with hot water) basalt to various extents. In the bottom figure (b), magma reacts with regions containing water-bearing minerals (such as amphibole, amph, or phogopite, phl). In both cases, some magmas make the trip from the mantle to the surface without reacting with the crust; those are the Martian meteorites with lowest oxygen fugacity. The crust is assumed to be enriched in elements that concentrate in magma ("incompatible elements") compared to the mantle. |
Wet Crust
This work suggests that there is a large difference between the mantle and crust of Mars, just as there is on Earth. It might also indicate that there are big differences even in the mantle. Magmas built the crust of Mars over time, though there was much more igneous activity early (before about 4 billion years ago) than more recently. This crust was modified by water, both on the surface and at depth. These modifications (making hydrous minerals, increasing the oxygen fugacity) themselves appear to have modified other magmas as they traveled through the crust. The study of these interactions is only beginning. More meteorite studies are being done, and present and future missions to Mars will help us understand the nature of the crust and its formation.
|
|
[ About PSRD |
Archive |
Search |
Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |