|
|
|
|
|
|
|
posted August 22, 1997 |
 (NASA photo) (NASA photo) | |
| The Martian Interior Written by G. Jeffrey Taylor Hawai'i Institute of Geophysics and Planetology |
The Viking missions in the mid-1970s and the recent Mars Pathfinder mission have shown us what the surface of Mars is like at three places. Their instruments have revealed the chemical composition of the surface and individual rocks. Exciting as those missions were, they literally only scratched the surface.
 | Sojourner's Alpha Proton X-Ray Spectrometer (APXS) examined rocks and soil on Mars. Here the rover is atop Mermaid Dune. (Image MRPS #82938, taken on Mars Sol 30, NASA; see enlargement.) |
We have samples of igneous rocks from Mars, in the form of the SNC meteorites. They were blasted off by impacts millions of years ago, and eventually made their way to Earth to fall as meteorites. (The best evidence that they come from Mars is that two of them contain trapped gas with a composition matching that of the atmosphere of Mars as measured by the Viking landers; see Meteorites from Mars at the Johnson Space Center.) Of the 12 Martian meteorites, all of which solidified near the martian surface by crystallization from a cooling magma, the most useful probes of the interior are the shergottites (the S in SNC). How do chemical analyses of martian meteorite samples tell us about the planet's innards?
Magmas originate inside planets, but their compositions are different from the rock that melts to produce them. Some elements are readily incorporated into magmas, while others stay behind in the unmelted portions of the mantle. As the magmas ooze their way to a planet's surface, other things can happen to them, such as partial crystallization in magma chambers beneath volcanoes, further changing their compositions.
In spite of this complicated chemical behavior, the abundances of elements in magmas contain a wealth of information about the composition of the region of the mantle in which they formed. The key to decoding this information lies in knowing that not all elements are greatly affected by melting and crystallization. Many elements that concentrate in the magma behave chemically alike, so the ratio of one to another does not change. It's as if magmas retain a detailed memory of their birth. But the decorder key works only if you can determine the composition of a magma.
 |
The martian meteorites allow us to do just that...to analyze the compositions of martian magmas. This basaltic shergottite meteorite from Mars, named Zagami [Data link from Meteoritical Database], has a mass of 18 kilograms and was observed to fall in Nigeria, Africa and recovered in 1962 (see enlargement). Samples like Zagami are investigated in great detail in geochemical laboratories around the world. |
Chemical Make-up
While several approaches have been tried to solve the puzzle of the martian interior, the one generally considered to be the best guess was made in the mid-1980s by G. Driebus and H. Wanke of the Max Planck Institute in Mainz, Germany. Their basic approach was to measure the abundances of a few key compounds, such as FeO (iron oxide), in shergottite samples, and then make use of averages and element ratios to estimate the abundances of the elements in Mars itself.
Iron is an extremely important element because it occurs as FeO in planetary mantles and as metallic Fe in cores. (It also occurs in more oxidized form (Fe2O3) on the surfaces of planets, appearing reddish. However, the amount of Fe2O3 is too small to worry about when computing the composition of an entire planet.) FeO does not concentrate strongly into magmas when they form, nor does it stay behind preferentially in the unmelted solids. Its abundance in a magma, therefore, is not much different from its abundance in a planet's mantle. The average FeO in five shergottites is 18.9 wt.%, implying that the martian mantle contains roughly that amount.
Driebus and Wanke were not satisfied with that rough estimate. They also noted that the MnO (manganese oxide) abundance in shergottites averaged 0.48 wt.%, about the same as in carbonaceous chondrites. They used this to make the bold assumption that the mantle of Mars has exactly the same MnO content as carbonaceous chondrites. Then, dividing the ratio of FeO/MnO in the shergottites (39.5) by the same ratio in carbonaceous chondrites (100.6), they estimate that the FeO content of the martian mantle is 0.39 of the FeO content of the chondrites. This translates to an abundance of FeO of 17.9 wt.% in Mars.
 |
This example of a carbonaceous chondrite was collected in the Grosvenor Mountains, Antarctia. GRO 95577 [Data link from Meteoritical Database] is 6.4 x 3.6 3.3 cm in size and weighs 106.2 grams (see enlargement). |
 Why would Driebus and Wanke think that carbonaceous chondrites had anything to do with Mars? Because these dark, carbon-rich, grundgy meteorites have chemical compositions very similar to the Sun (except for the most gaseous elements such as hydrogen and helium), they are thought to represent the average stuff that makes up the Solar System, including the planets. The chondrites form a useful basis for comparison.
Why would Driebus and Wanke think that carbonaceous chondrites had anything to do with Mars? Because these dark, carbon-rich, grundgy meteorites have chemical compositions very similar to the Sun (except for the most gaseous elements such as hydrogen and helium), they are thought to represent the average stuff that makes up the Solar System, including the planets. The chondrites form a useful basis for comparison.
By making numerous other comparisons of element abundances, Driebus and Wanke were able to deduce the concentrations of the most abundant elements in Mars, and some less abundant ones too (see table below). A comparison with the composition of the Earth's mantle (which is much better known) shows that Mars contains much more FeO than does the Earth.
| Compound | ||
| SiO2 | ||
| TiO2 | ||
| Al2O3 | ||
| Cr2O3 | ||
| MgO | ||
| FeO | ||
| MnO | ||
| CaO | ||
| Na2O | ||
| K2O | ||
The composition and size of the metallic core is also important. The larger it is, the smaller the depth and lower the pressure at the boundary between the mantle and core. Driebus and Wanke estimated the composition of the martian core by assuming that the total composition of Mars is the same as carbonaceous chondrites and that all the iron and nickel not present in the mantle are in the core as metallic Fe. They also assumed that the core contains all the sulfur in the planet. This gives a composition of the martian core of 77.8 wt.% iron, 7.6 wt.% nickel, and 14.2 wt.% sulfur.
Although the high FeO composition of the martian mantle is generally accepted by planetary scientists, not all agree. In particular, S. Ghosal, R. O. Sack, and M. E. Lipschultz of Purdue Univeristy, and M. S. Ghiorso of the University of Washington in Seattle, argue on the basis of mineral compositions in martian meteorites that conditions in the martian mantle were much less oxidizing than generally assumed. This would produce more metallic iron in the core, and less oxidized iron (FeO) in the mantle. Using a computer program called MELTS [link] that simulates melting and crystallization of magmas, they estimate that the martian mantle contains only 6.8 wt.% FeO, even lower than that of the Earth. Their work, to be published in Contributions to Mineralogy and Petrology, will be hotly debated when it appears in print. PSR Discoveries will follow the debate as it unfolds.
So, now we have estimates of the chemical composition of the Martian interior. But what about the minerals present? This is where Bertka and Fei enter the story.
To know the physical state of materials at the high pressures and temperatures existing inside planets, you must simulate those conditions in the laboratory. This requires some highly specialized equipment that can compress and heat samples, and then allow them to be recovered for study.
 |
Bertka (in photo, see enlargement) and Fei used a device called a multi-anvil press. It is made of a sturdy housing that contains six removable wedges. The wedges transmit forces from a hydraulic piston to the interior of the device. Inside there is a cube made of eight separate tungsten carbide cubes, each with a corner cut off. The truncated corners meet in the center, forming a region shaped like an octahedron. This is where the high pressures are attained during an experiment. A small furnace (less than 1 cm in diameter) containing the sample is placed in the octahedral area inside the multi-anvil press. The samples Bertka and Fei used were a mixture of oxide powders with overall compositions identical to the calculated composition of the martian mantle. Once assembled, the furnace could be controlled to give the desired temperature, and the pressure controlled by the force applied by the hydraulics. The experiments lasted between 3 and 48 hours at temperatures of 1100oC to 1765oC and pressures ranging from 20,000 to 235,000 times the pressure at the surface of the Earth. These conditions correspond to depths of 200 to 2000 km inside Mars. (See note about pressure.) |
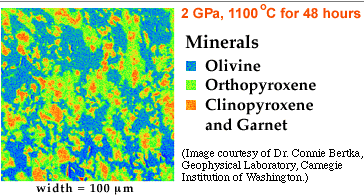
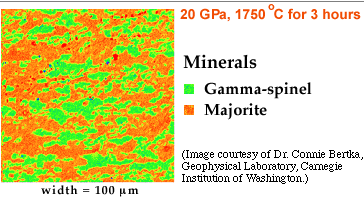
The results show that the minerals change as pressure increases, even though the chemical composition of the material remains the same. Not surprisingly, at higher pressures, minerals with higher densities form. The mineral olivine, no longer stable at the higher pressure (20 GPa), converts to a crystal form called gamma-spinel. The pyroxenes and garnet convert to majorite. These mineralogies are illustrated in the false-color images below.
| Electron microprobe image of an experimental product colored according to chemical composition. Pressure was 2 GPa. Temperature was 1100oC. The experimental run lasted for 48 hours. |
 |
| In this case, the pressure was 20 GPa. Temperature was 1750oC. The experimental run lasted for 3 hours. |
 |
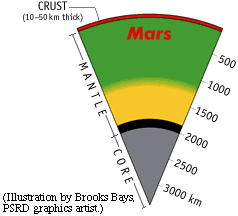 |
The experiments of Bertka and Fei give us one possible picture of the interior of Mars (see enlargement). In this picture, the uppermost mantle of Mars consists of olivine and pyroxene, with a small amount of garnet (shaded green). These are fairly common minerals on Earth, the other planets, the Moon, and asteroids. However, at a depth of about 1100 km, the olivine begins to convert to a more dense form, called gamma-spinel, without changing its chemical composition. The conversion is complete by 1300 km. Along with the conversion of olivine to a spinel crystal structure, garnet and pyroxene convert to a mineral called majorite, which has a crystal structure like garnet, but is close to pyroxene in chemical composition (shaded yellow). At higher pressures, hence deeper, there is a relatively abrupt transition at 1850 km (shaded black) to a mixture of perovskite (itself a mixture chemically of MgSiO3 and FeSiO3) and magnesiowustite (a mixture of FeO and MgO). The metallic core (shaded gray) begins at about 2000 km depth and continues to the center at a depth of 3390 km. |
Of all the features in Bertka and Fei's picture, the presence of the thin, lowermost mantle layer is most uncertain. If the core is cooler than Bertka and Fei assume, then the layer may not exist. It's presence may affect the formation of zones of mantle upwelling, called plumes, that could have produced widespread volcanism on Mars. The dynamics of plume formation is dependent on the nature of the lower mantle, so this is an important matter to settle.
Testing the Model
How can we test the picture Bertka and Fei have produced of the Martian interior? The most direct test would be to install a network of seismometers on the surface of Mars, to record marsquakes. This would show how seismic wave velocities vary with depth, from which the density and temperature profiles could be established. The data would also allow measurement of the size of the core and thickness of the crust. Mars missions, including Pathfinder, will also improve estimates of a parameter called the moment of inertia factor, which provides information about the distribution of mass inside a planet. This parameter is not known with certainty now, so different estimates for the composition of the interior of Mars cannot be tested. Once we have seismic data and a better determination of the moment of inertia factor, it will be possible to decide if the high-FeO mantle favored by Dreibus and Wanke is correct, or whether the low-FeO mantle composition recently proposed by Ghosal and coworkers is more reasonable.
A global network of seismometers would also show how the mantle of Mars varies horizontally. When this is compared to Bertka and Fei's internal structure, any variations might be due to motions in the mantle, perhaps associated with incipient plate tectonics, or rising plumes that drove volcanism.
 | The Mars Pathfinder Sojourner rover analyzed the rock called "Yogi" on Mars Sol 6. (Image no. 81314, NASA.) A seismic network around Mars could someday be installed by rovers. Thus, Mars Pathfinder is an important first step in the exploration of the surface and interior of Mars. |
|
|
[ About PSRD |
Archive |
Search |
Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |