|
|
|
|
|
|
|
Recipe for High-Titanium Lunar MagmasWritten by G. Jeffrey TaylorHawai'i Institute of Geophysics and Planetology |
|
Lunar volcanic glass deposits contain information about the composition of the Moon's interior and the melting processes that operated in it. Some glasses have unusually high amounts of titanium (expressed as titanium oxide, TiO2), up to about 16 wt%. All hypotheses for the formation of these high-titanium magmas make use of the presence of a titanium-rich layer. This unusual layer was produced by the crystallization of a huge ocean of magma that surrounded the Moon when it formed. Experiments indicate that this layer would consist mostly of ilmenite (FeTiO3) and a variety of the mineral pyroxene that is rich in calcium.
In one hypothesis the ilmenite-pyroxene layer sank and mixed with low-titanium rocks inside the Moon. This produced a hybrid rock that later melted to form high-titanium magma. In a second hypothesis, low-titanium magma oozes upwards in the Moon and reacts with the ilmenite-pyroxene layer, producing a magma high in titanium. James Van Orman and Timothy Grove (Massachusetts Institute of Technology) question both ideas. Their skepticism is based on the density and ease of flow of the ilmenite-pyroxene layer and on the rates of dissolution of ilmenite and pyroxene. To quantify their arguments, they performed a series of experiments. In one set, they determined the order of crystallization in the ilmenite-pyroxene layer. In the other series of experiments they measured the rate at which ilmenite and pyroxene dissolve in low-titanium magmas. Their work leads them to propose a third hypothesis that calls on shallow (just below the lunar crust) reaction and mixing of the molten ilmenite-pyroxene layer with underlying solids rich in the mineral olivine. This hybrid material would be able to sink readily, and eventually remelt deep inside the Moon.
Volcanic glasses
"It's orange!" astronaut Harrison (Jack) Schmitt exclaimed from the rim of Shorty Crater. Schmitt, the only geologist to explore the Moon in person, was doing lunar fieldwork with Eugene Cernan on a sunny day in December 1972. They had discovered a deposit of orange soil. The soil, known ever since as "the Apollo 17 orange soil," had been buried beneath lava flows until an impact 30 million years ago formed Shorty Crater. The impact blasted a crater 110 meters across and about 20 meters deep.
 |
Apollo 17 astronauts found unusual orange soil on the rim of Shorty crater. |
Schmitt went to work sampling the orange soil with a scoop and by hammering a pipe into the surface. When pulled out, the pipe contained 71 centimeters of soil. Most important, the pipe preserved the layering in the soil. Two days later Schmitt and Cernan blasted off from the Moon, carrying 110.5 kilograms of rock and dirt with them, including the carefully selected samples from Shorty Crater. Scientists began to study samples of the orange soil soon after Apollo 17 safely splashed down in the Pacific. They discovered that it was composed of a bunch of orange and black spheres. The orange ones were made of glass rich in TiO2. The black ones were partly crystallized; because one of the minerals in them was opaque, the glass appears black.
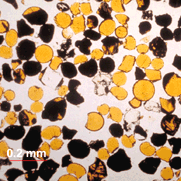 |
Here is a thin slice of some Apollo 17 orange glass spheres as viewed through a microscope. The color is caused by the presence of about 8 wt% TiO2. (Photographed by Graham Ryder, Lunar and Planetary Institute.) |
There was a vigorous debate about the origin of the orange soil. One group suggested that the glass spheres were formed by meteorite impact. They pointed out that the lunar surface was affected severely by impact and that there were plenty of impact-generated particles in the typical soil. Another group argued that the orange soil was a volcanic deposit formed when fountains of lava spouted from the ground and fragmented into millions of droplets when gases escaped. Such fountains are common during volcanic eruptions on Earth.
Gases shoot lava high into the air during eruptions on Earth, like this one at Volcano in Hawai'i. On the Moon, droplets of lava would have traveled far from the vent because of lower gravity and no air on the Moon. |
 |
Grant Heiken and David McKay of the Johnson Space Center in Houston began to settle the issue in 1974. (Heiken is now at Los Alamos National Laboratory.) They studied the layering in the core tubes and found that it was very similar to deposits made by volcanic fountains on Earth. They also noted that the surfaces of the orange glass beads contained deposits of elements and minerals that are gaseous during volcanic eruptions.
 |
Orange glass spheres contain deposits of volatile substances. The faceted ones, in this black and white view using a scanning electron microscope, are sodium chloride (table salt). The bumpy surface layer is composed of other elements, including lead and sulfur. These deposits are typical of lunar volcanic glasses. |
John Delano (State University of New York, Albany) spent years developing additional criteria to identify volcanic glasses. It was especially important to do this because most samples were found in soils that had been mounted on glass slides and ground into thin slices. This made it impossible to study their surfaces. Delano devised an impressive list of features that a glass ball had to meet to qualify as volcanic.
Delano's criteria involve the appearance and composition of a sample. Volcanic glasses lack dusty mineral grains that are common in impact glasses. Instead, they are homogeneous in appearance. They also have homogeneous chemical compositions because individual pieces of hot magma are chemically uniform. In contrast, impact melted droplets do not stay hot long enough to homogenize chemically. Volcanic glasses cluster in their chemical compositions. This happens because magmas are surprisingly uniform in composition over large distances, so dispersed droplets are also uniform in composition. In contrast, clots of impact melts vary much more in composition because they form by melting of heterogeneous targets. Finally, lunar volcanic glasses have a high ratio of magnesium (Mg) to aluminum (Al). This esoteric criterion is surprisingly useful. The two certified volcanic glass deposits (Apollo 15 green and Apollo 17 orange) have high Mg/Al. Remote sensing measurements indicate that large deposits of volcanic glass have higher Mg/Al than typical lunar surface materials.
Why the big fuss about whether a glass formed by volcanism or impact? Simple: lava forms inside a planet, so its composition reflects both the chemical and mineralogical make up of the interior and the processes that operate inside the planet. Like a doctor's blood sample, lava tells you what goes on inside a planet. The quest for this important information led John Delano to find and analyze thousands of volcanic glass spheres. He discovered that they had a striking range in chemical composition. This is shown most prominently by the amount of TiO2, which varies from 0.6 wt% to 16 wt%. This compositional change is accompanied by a change in color from green to yellow to orange to red. The red glasses were what interested Jim Van Orman and Tim Grove.
Red volcanic glasses
The startlingly high TiO2 concentrations in the red volcanic glasses has led to some dramatic tales for how they formed. The lunar magma ocean plays an important role in most of the yarns. The idea the infant Moon was surrounded by a layer of magma hundreds of kilometers thick is a central tenet of our ideas about the Moon's evolution. As the magma ocean crystallized, dense minerals sank and lighter minerals floated. Feldspar was the main floater, and it accumulated into a pile tens of kilometers thick to form the original lunar crust. Iron and magnesium silicates like olivine and pyroxene sank; they would later play a role in making lunar basalts.
By the time 90% of the magma ocean had solidified, it was loaded with titanium, so iron-titanium oxide (ilmenite) began to form. Ilmenite crystallized with calcium-rich pyroxene and they accumulated into a layer beneath the crust. The layer would have been about 20 kilometers thick. Both are dense minerals, so this layer would have a tendency to sink. It would also have a tendency to chemically react with magmas low in titanium. The tendencies to sink or react are parts of the stories scientists tell about the origin of the red glass magma. Of course, we do not actually know for sure if a titanium-rich layer formed or, if it did, that it sank. However, most lunar scientists are pretty sure about the magma ocean. [See PSRD articles: New Moon for the Twenty-First Century and The Surprising Lunar Maria.]
 |
Dense, titanium-rich rock might have sank through the underlying rock in the lunar mantle. The titanium-rich layer might also have reacted chemically with low-titanium magma to form new magma with a high concentration of titanium. |
Experiments conducted by several researchers during the 1970s and 1980s showed that the magma that erupted to produce the red glasses formed about 400 kilometers beneath the lunar surface. Although there are some uncertainties in this, most Moon experts agree that the red glass magmas came from deep inside the Moon. The experiments also showed that the red glass could not have formed by melting of the ilmenite layer directly.
![]() There are two schools of thought for the origin of red glass magma. In one, low-titanium magma forms deep inside the Moon. The magmas migrate through the mantle and eventually encounter the ilmenite layer below the crust. In this case, in spite of its high density, the ilmenite layer had not sunk. The low-titanium magma reacts with the titanium-rich rock, producing a magma with a high titanium content. A major problem with this story is that it may alter the chemical composition too much to make magma like the red glasses. In particular, ilmenite and pyroxene must melt and become part of the magma in the ratio of 3:1. However, ilmenite would have been much less abundant than pyroxene in the high-titanium layer: they would have occurred in the proportions of 1:5 or 1:6. Perhaps, Tim Grove thought, ilmenite dissolved faster in the low-titanium magma than did pyroxene. There was a lack of experimental data to test this idea. Hence, he and Jim Van Orman planned a set of experiments to see how fast ilmenite and pyroxene melt.
There are two schools of thought for the origin of red glass magma. In one, low-titanium magma forms deep inside the Moon. The magmas migrate through the mantle and eventually encounter the ilmenite layer below the crust. In this case, in spite of its high density, the ilmenite layer had not sunk. The low-titanium magma reacts with the titanium-rich rock, producing a magma with a high titanium content. A major problem with this story is that it may alter the chemical composition too much to make magma like the red glasses. In particular, ilmenite and pyroxene must melt and become part of the magma in the ratio of 3:1. However, ilmenite would have been much less abundant than pyroxene in the high-titanium layer: they would have occurred in the proportions of 1:5 or 1:6. Perhaps, Tim Grove thought, ilmenite dissolved faster in the low-titanium magma than did pyroxene. There was a lack of experimental data to test this idea. Hence, he and Jim Van Orman planned a set of experiments to see how fast ilmenite and pyroxene melt.
![]() The other school of thought involves overturn of the entire pile of magma-ocean products. The ilmenite layer sank, the lowest layers rose, and everything got all mixed up. Melting of these hybrid rocks produced a variety of magmas, with a huge range in titanium concentrations. This story hinges on when the ilmenite layer sank . While still molten, it would be less dense than the solids beneath it, so at least some of it must crystallize before it is possible to sink. However, if too much has to crystallize to attain the correct density, it might not be fluid enough to sink. This led to a second set of experiments by Van Orman and Grove.
The other school of thought involves overturn of the entire pile of magma-ocean products. The ilmenite layer sank, the lowest layers rose, and everything got all mixed up. Melting of these hybrid rocks produced a variety of magmas, with a huge range in titanium concentrations. This story hinges on when the ilmenite layer sank . While still molten, it would be less dense than the solids beneath it, so at least some of it must crystallize before it is possible to sink. However, if too much has to crystallize to attain the correct density, it might not be fluid enough to sink. This led to a second set of experiments by Van Orman and Grove.
Heating and squeezing to test ideas about red glass magma
Van Orman and Grove did two sets of experiments. In one set they concocted a mixture of chemicals with the composition expected for the ilmenite layer. They wanted to determine how the abundance and composition of crystals and left over magma varies as the temperature falls. In the second set of experiments, they measured the rate at which ilmenite and pyroxene dissolved in magma with the composition of low-Ti basalt.
 |
Undergraduate students prepare samples for experiments in Tim Grove's lab at MIT. The students were involved in the Undergraduate Research Opportunity Program, an MIT invention during the 1960s. It is a model for how students benefit from studying at a research university. |
 |
Low-pressure experiments were done in this furnace. Van Orman and Grove suspended powders fused to wire loops made of iron and platinum in a tube-shaped oven located behind the white panel. They used iron-platinum because iron dissolves readily in many metals, but less so in that alloy. Van Orman and Grove controlled the atmosphere flowing through the furnace so it would have oxidizing conditions like those in lunar magma. The tubes leading into the top of the furnace feed the gas mixture. The electronics to the left control the temperature and flow rate of the gases. |
 |
Because the ilmenite-rich layer was beneath the lunar crust, some experiments had to be done at a pressure appropriate a magma residing under many kilometers of rock. Van Orman and Grove chose a pressure a bit over 10,000 times the pressure at the surface of the Earth. This is a photograph of a high-pressure rock squeezer that uses a small piston to smash the sample from one end against a flat plate. The experiment uses small pellets (about 10 milligrams) of synthetic rock powder. Each pellet was placed into a graphite container, which was placed inside a platinum outer capsule. This package was placed into the furnace, where it was squeezed and heated. Crystallization experiments lasted 10 to 24 hours. Mineral dissolution experiments lasted 24 to 148 hours. |
The crystallization experiments show what minerals are present at different temperatures. They also show how much of each mineral and magma are present at a given temperature. A curious thing about magma and other complex compounds is that they do not solidify at a single temperature. One mineral forms first, followed by a sequence of other minerals. In the case of the red glass magma, pyroxene forms first. As the temperature drops, ilmenite joins pyroxene. This is followed by an interval in which those minerals continue to crystallize but are joined by quartz. The key point is that most of the crystallization takes place very close to the point at which the magma becomes totally solid. If the temperature were only 30 degrees Celsius above that temperature (at any pressure), the ilmenite-rich layer would be more than 50% molten.
This changes the behavior of the hypothesized ilmenite-rich layer. As explained above, some hypotheses for making high-titanium magma call on the ilmenite-rich layer to sink as a solid, dense mass. However, if it is largely liquid, it will have a lower density that the underlying rock. It will not sink. Van Orman and Grove also show that if the ilmenite-rich layer is almost solid, it will be too cool to flow easily: it will be too stiff to sink. So, the ilmenite-rich layer is either not dense enough to sink, or dense enough but too stiff to sink. This ruins the whole idea of mare basalt magma forming from mixed sources.
What about the idea of low-titanium magma reacting with the ilmenite-rich layer? The dissolution and melting experiments do not help that idea. Van Orman and Grove did two types of experiments. In one set, they measured the rate at which ilmenite and pyroxene melted when in contact with each other. To do this, they obtained gem quality crystals of each mineral, cut the samples into thin slabs, polished the surfaces, and stuck them together. By heating these mineral couples at high pressure and measuring the thickness of each mineral slab before and after the experiment they could determine how much dissolved. That amount divided by the time gives the rate of dissolution.
 |
Electron microscope image of an ilmenite-pyroxene experimental pair after heating for 1 hour at 1350 degrees Celsius at 13,000 atmospheres. The zone between the ilmenite and pyroxene is now glass, but was magma during the experiment. Little chunks of pyroxene occur in the glass because of melting along cracks. (The glass does not have the composition of the red glass magma, so simple partial melting of the ilmenite-pyroxene layer would not produce the red glass magma.) |
The experiments show that the rates of ilmenite and pyroxene melting depend on temperature. At the temperature at which the ilmenite-pyroxene layer would just begin to melt, about 1200 degrees Celsius, pyroxene melts ten times faster than ilmenite. At the temperature at which the red glass magma is totally molten, about 1400 degrees Celsius, ilmenite and pyroxene melt at about the same rate. At the high temperature they melt very fast: centimeter-sized grains will melt in only a few hours.
In the other set they measured the rate at which pyroxene dissolved into magma with a composition of low-titanium basalt. (The rate of ilmenite dissolution had been measured previously by Tim Grove and T. Wagner.) The results show that ilmenite dissolves faster than pyroxene at low temperature (about 1200 degrees C). At the higher temperatures like those of red glass magma, however, both minerals dissolve at about the same rate. These dissolution rates are about 3 times lower than the rates at which pyroxene and ilmenite melt when in contact with each other.
So, what does this mean for forming the red glass magma by reaction with the ilmenite-pyroxene layer? It means it is unlikely to have been the source of the titanium in the red glasses. For it to work by partially melting the layer and mixing it into a low-titanium magma, more ilmenite must enter the mixed magma than does pyroxene. In fact, the ratio needs to be about 3:1. But the experiments show that pyroxene dissolves more rapidly than ilmenite, not less rapidly. At high temperature both dissolve at the same rate, but this rate is so high that both would be incorporated easily. Because pyroxene is more abundant than ilmenite in the ilmenite-pyroxene layer, the resulting magma would not contain anywhere near the needed 16 wt% TiO2. Overall, the experiments indicate that it is unlikely that reaction of the ilmenite-pyroxene layer with any kind of magma would have produced a magma with the compositional characteristics of the red glasses. Since the crystallization experiments seem to have ruled out sinking of the ilmenite layer, it appears that Van Orman and Grove showed that all stories about how the red glass magma formed were fables. But they do exist, so how did they form?
Add a pinch of olivine to the recipe
Van Orman and Grove concentrate on ways to make the ilmenite-pyroxene layer sink. They do not think it will do so without somehow making it denser. They suggest that late in the solidification of the magma ocean the titanium-rich layer of magma would not solidify simply into the hypothetical ilmenite-pyroxene layer. Instead, as it was forming it would react with other minerals already formed. In particular, Van Orman and Grove suggest that olivine, the most abundant mineral formed from the magma ocean, was incorporated into the ilmenite-pyroxene layer. As little as 20% olivine would raise the melting temperature of the ilmenite-pyroxene by about 80 degrees Celsius. The higher temperature would make the layer flow much easier, allowing it to sink. The addition of 20% olivine would decrease the density of the layer a bit, but it would still be more dense than the underlying rocks, so would sink. Once it sank, the ilmenite-pyroxene layer would be able to mix with other rocks deep in the interior to make any type of lunar basalt magma you want.
Van Orman and Grove argue that this initial mixing before sinking may be a natural consequence of the late history of the magma ocean. Complete crystallization of the ocean might have taken 200 million years, or more, allowing lots of time for mixing in its upper layers. In addition, recent observations that radioactive elements are concentrated in one region of the Moon, [See PSRD article: New Moon for the Twenty-First Century] may have caused the magma ocean to take even longer to finish solidifying.
This work shows the interplay among the many ways we study planetary materials. The story of the red glass magma needed careful field observations and sampling on the Moon; development of criteria to identify volcanic glasses; loads of data on lunar samples; imaginative ideas for what those data mean in terms of how the red glass magma formed; and experiments to test the ideas.
|
|
[ About PSRD |
Archive |
Search |
Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |