|
|
|
|
|
|
|
Whether the interesting features in martian meteorite ALH 84001, Data link from Meteoritical Database, (see the October, 1996 Hot Idea of PSR Discoveries) prove that life existed on Mars or are the products of nonbiological processes is being hotly debated, and will continue to be argued for at least the next few years. The debate raises some intriguing questions about life on Mars, if it exists. How did it begin? Where on the planet did it begin? Could life still exist now, perhaps in water-bearing rocks below the surface? Recent research on deep rocks on Earth has shown that bacteria can live kilometers beneath the surface. Some bacteria live on nothing but rock and water, extracting energy from chemical reactions rather than from sunlight. Life on Earth, and perhaps on Mars and even other planetary bodies may have originated in such strange environments, and if so, the subsurface of water-rich planets, asteroids, and satellites might be home to a rich diversity of microorganisms.
 |
|
Other planetary surfaces do not teem with life like we see on Earth. We know this from images taken by landers and, in the case of the Moon, by direct observations. On the other hand, we know almost nothing about life beneath the surfaces of other planets. In fact, we have only begun to explore life underground on our own planet and in other odd places, such as the ocean floor. It turns out that there is a vast ecosystem of microorganisms underground, and much of this system does not depend on sunlight and photosynthesis. Some of these microorganisms live off the rocks that house them. |
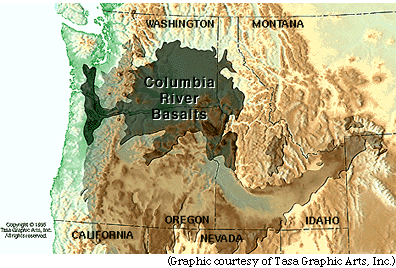 |
Todd O. Stevens and James P. McKinley of the Pacific Northwest Laboratory (Richland, Washington) have been studying groundwater in the Columbia River basalts, located in the northwestern U.S. mainland (see map). |
Stevens and McKinley began their work as part of a project managed by the U.S. Department of Energy to thoroughly understand how contaminants are transported underground, so scientists and engineers can devise ways to control pollution from nuclear and chemical waste facilities. It is possible that bacteria living underground could trap contaminants. Their research involves analyzing water obtained from wells at various depths down to about 1.5 kilometers in the Columbia River basalt sequence. Their initial results were published in the October 20, 1995 issue of the journal Science.
 |
The Columbia River basalt province consists of a huge thickness, up to a few kilometers; individual lava flows are typically tens of meters thick. Most of the lavas erupted between 17 and 13 million years ago. This photograph shows layered lava flows each 30-50 meters thick at Dry Falls, Washington. The groundwater aquifers are located at the boundaries between individual flows. |
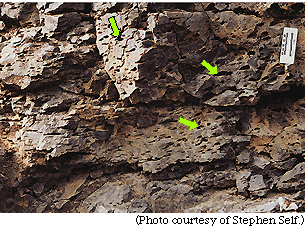 |
The tops of individual lava flows contain abundant holes, called vesicles, which can be filled with water in underground aquifers. A representative flow top with vesicles (dark spots) in a Columbia River basalt is shown in this photograph taken on the surface at Banks Lake, Washington. Arrows point to some of the largest vesicles. |
Water also can flow through cracks and other types of small cavities. The water in the wells studied by Stevens and McKinley has resided in the rocks for a long time, up to 35,000 years, judging from the very low content of carbon-14 (which is produced in the atmosphere). As a result, the water contains essentially no dissolved oxygen. (The water, of course, contains one oxygen atom for every two hydrogen atoms in its basic chemical structure. But water can also dissolve other substances, including gases such as oxygen and carbon dioxide.) The low carbon-14 and dissolved oxygen contents are not found in water sources near the surface, showing that the deep well water has not been contaminated by shallower water as it is pumped to the surface.
Analyses of the well water show that it contains surprisingly high amounts of dissolved hydrogen gas, H2 and methane, CH4. Cultures of the water also demonstrated the presence of several types of bacteria. Stevens and McKinley suspected that the H2 was made by the reaction of the oxygen-poor water with iron-bearing minerals in the rocks. Prime candidates are pyroxene and olivine, minerals rich in iron and common in basalts. The reaction would produce iron in a more oxidized state, making minerals such as magnetite.
In their scheme, the methane was made by bacteria that used the hydrogen and carbon dioxide in the water. High methane contents were associated with enrichments of carbon-12 over carbon-13. This change in isotopic ratio is characteristic of methane produced by bacteria during the reduction of carbon dioxide, supporting their interpretation. Stevens and McKinley also devised two experiments to see if these reactions would take place and if bacteria could live in an environment consisting of oxygen-free water and rock.
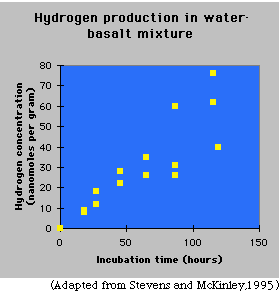 |
Stevens and McKinley mixed sterilized water that contained no dissolved oxygen with sterilized crushed basalt, and sealed the mixture in glass tubes. They placed the experimental packages in the dark for a few to 120 hours. To be sure that the basalts were not contaminated with steel, which would be oxidized by the water, they crushed the rock with ceramic tools. |
The graph shows the results of the experiment. The hydrogen content of the water became higher the longer the sample sat in its dark, wet environment, showing that the water reacted with the rock. The scatter in the graph is common in scientific experiments, and statistical analysis shows that the trend of increasing hydrogen with time is significant. Stevens and McKinley also did the experiment with iron-poor rocks such as granites and sandstones, and no significant amount of hydrogen was produced.
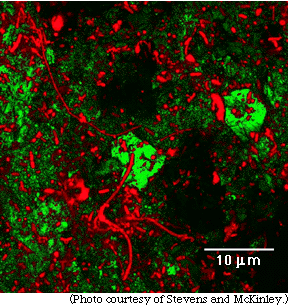 |
| Photographs, above and below, were taken with a special microscope called a scanning laser confocal microscope. The laser reflects off the rock surfaces and shows up green. The bacteria were dyed with a chemical and appear red in the laser light in both photographs. |
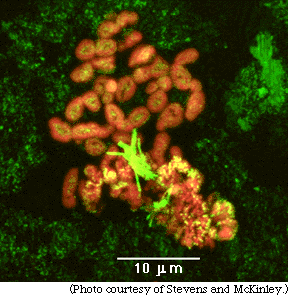 |
| The types of bacteria shown in red in these two photographs have not yet been identified (and may be previously unknown species), but the experiment clearly shows that bacteria can live deep underground if deoxygenated water and iron-bearing minerals are present. |
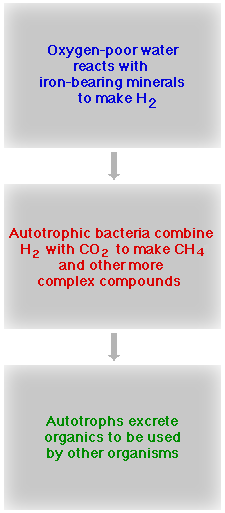 |
This diagram summarizes what appears to be happening below the surface inside the Columbia River basalts. The oxygen-poor water reacts with iron-bearing minerals such as olivine and pyroxene to make H2. Bacteria use the H2 and dissolved CO2 to make methane (CH4) and other hydrocarbon molecules needed to build cell material. Bacteria that use inorganically-produced chemicals to make organic chemicals are called autotrophs, or in this case, because they use rock in the process, lithoautotrophs. The autotrophic bacteria then excrete organic chemicals that are used by other microorganisms. Stevens and McKinley call this partnership a Subsurface Lithoautotrophic Microbial Ecosystem, or SLiME for short. |
 | |
|
The SLiME system discovered by Stevens and McKinley is not the only case of life either underground or far from sunlight. Deep sea hydrothermal (hot water) vents are teeming with life, as are buried sediments. In an article in the July, 1992 issue of the Proceedings of the National Academy of Sciences, Thomas Gold (Cornell University) has tried to calculate the amount of subsurface life in comparison with the above ground life all around us. His estimate necessarily contains some guesses, but all are reasonable. Assuming that the upper 5 kilometers of Earth has a porosity of 3% (porosity is the amount of space available for water in a rock or sediment), and that 1% of the mass of the water filling the pore spaces was bacteria, then the total mass of bacteria would be 200 trillion metric tons. Putting it another way, Gold points out that this is equivalent to a layer on the order of 1.5 meters high covering the entire Earth! This is more than the existing plant and animal life on the surface, which is estimated to be about a trillion metric tons. Even if Gold's estimate is off by a factor of 100, the amount of subsurface life is at least equal to that on the surface. |
When we explore Mars, water- and organic-rich asteroids, the icy satellites of the outer planets, and Pluto, we may find that some of them, in spite of barren, apparently lifeless surfaces, are teeming with life underground. If life exists on Mars today, we can guess where it could be found. Bacteria require liquid water at least some of the time, so the temperature cannot be permanently below freezing. Present day Mars has permanently frozen ground near the surface, perhaps down to a depth of a few kilometers, so life is unlikely to be flourishing there. The melting temperature of ice is reached at a depth of about 10 km, so life could exist at that depth. However, it cannot be so deep that the temperature is too hot to allow bacteria to survive. Boiling water (100o C) will kill most bacteria, but experiments have shown that bacteria can live at 115o C, and some biologists speculate that bacteria can survive up to 150o C at high pressures (which prevents water from boiling). These temperatures correspond to a depth of about 20 km on Mars, though this is highly uncertain. So, if life exists on Mars today, the most likely place where it could be found is in a small region between 10 and 20 km beneath the surface.
One of the surprising facts about the possible discovery of fossil life in martian meteorite ALH 84001 is that it seems so incredibly unlikely to find that a random rock tossed to us by a random impact would contain evidence for life. The only likely way for such a random rock to contain fossilized microorganisms would be if such microorganisms were common and widespread under the martian surface, as they are underground on Earth.
The surface of Earth, with its trees, grasses, and flowers, its dogs, cats, field mice, elephants, and us, may be the unusual place, decorated with a little bit of life on its surface. Underground, Earth may not be much different from other planets. Clearly, close inspections of the subsurfaces of other planetary bodies need to be done before we can know if we are alone.
|
|
[ About PSRD |
Archive |
Search |
Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |