|
|
|
|
|
|
|
Supernova Debris in the Solar System |
 |
Meteorites contain clear evidence that isotopes with short half lives (as short as 100,000 years) were present in the cloud of gas and dust (the called solar nebula) from which the Sun and planets formed. Supernovae, the powerful explosions of spent stars, produce elements, including short-lived radioactive isotopes. Given the short lifetimes, these elements must have been added immediately before solids formed in the Solar System, and it is possible that a supernova triggered the collapse of the vast interstellar cloud in which the Solar System formed. However, there is some evidence that two isotopes, aluminum-26 and manganese-53, were not distributed uniformly in the solar nebula. If correct, does this mean that the supernova debris was not mixed thoroughly into the collapsing interstellar cloud? This possibility was tested by Robert H. Nichols, Frank Podosek, and Cristine Jennings (Washington University in St. Louis) and Brad Meyer (Clemson University). They evaluated how thoroughly supernova products were mixed into the solar nebula by searching for the effects on the isotopic make up of other elements. They conclude that the explosive products of a supernova would have been mixed uniformly into the nebula. Thus, either the evidence of heterogeneous distribution of short-lived isotopes is incorrect, or some isotopes were not formed in a supernova, but came from somewhere else. This research project is one of many that link studies of meteorites, astronomical observations, and astrophysical calculations.
Isotopes That No Longer Exist
The atoms that make up the Sun, planets, asteroids, comets, and us formed in numerous places in the galaxy, mostly the interiors of stars and during supernova explosions. The products of the element-forming events are generally well mixed in the Solar System. There are some notable exceptions to this, such as the tiny grains of interstellar dust preserved in some meteorites [See PSRD article: Moving Stars and Shifting Sands of Presolar History.] Some of the isotopes were radioactive. Many, such as uranium-238 and potassium-40, exist in the Solar System today because their half lives are so long that they have not decayed completely away. Others had half lives so short that they no longer exist, and are identified only by variations in the abundances of the decay products. For example, aluminum-26 decays to magnesium-26. By analyzing ancient minerals rich in aluminum and poor in magnesium, cosmochemists can determine that the mineral contains more magnesium-26 than average Solar System material, indicating that aluminum-26 was present.
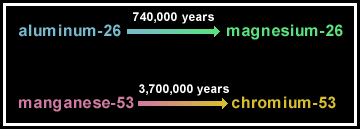
Many scientists have devoted much of their scientific careers to determining the initial abundances of now-extinct radioactive isotopes. It might seem to be an odd way to make a living, studying something that no longer exists, but the payoff in understanding element formation, the formation of the Solar System, and the timing of events during its formation is well worth the effort. Robert Nichols and his colleagues have used the abundances of extinct aluminum-26 and manganese-53 (which decays to chromium-53) to test the extent to which supernova rubbish was mixed into the interstellar cloud parental to the Solar System.
Although some scientists disagree, it is widely held that the same event that contributed short-lived isotopes to the Solar System was also responsible for triggering the collapse of the interstellar cloud that gave rise to the Solar System. The idea is that the elements must have been added very shortly (astrophysically speaking) before the cloud collapsed, and if a supernova was the source of the isotopes, then it is logical to call on the strong shock wave from it to induce the gravitational collapse of the interstellar cloud. The alternative is that radioactive elements were produced as a product of the formation of the Solar System itself (such as in intense radiation regions around the nascent Sun). To focus the problem, Nichols and colleagues assume that the isotopes were produced during a supernova.
Homogeneous or Heterogeneous?
A big debate centers on whether short-lived isotopes were distributed uniformly throughout the solar nebula. It would seem that an explosion would mix ingredients very efficiently, but the interstellar cloud was about a light year across before it began to collapse. (A light year is the time it takes light to travel in a year, about 10 trillion kilometers.) Some evidence for variations in the amount of supernova debris comes from the abundance of aluminum-26 in objects in meteorites called calcium-aluminum-rich inclusions (CAI for short).
CAIs are thought to be the first solid objects formed when the Solar System formed. Analysis of CAIs indicates that the ratio of aluminum-26 to the common, nonradioactive aluminum-27 was about 0.00005 when the objects formed. However, a few CAIs have much lower ratios, so low that they may not have contained any aluminum-26 at all. This might indicate that they formed later than the normal CAIs (the amount of aluminum-26 decreases by 50% every 740,000 years). This would mean that the solar nebula lasted at least several half lives of aluminum-26, or several million years, an interpretation that conflicts with other observations and theories depicting how long the solar nebula lasted. As a result, many scientists think that aluminum-26 was not distributed uniformly--some regions had more than others.
|
Mineralogical map of a calcium-aluminum-rich inclusion (CAI) in the Efremovka carbonaceous chondrite [Data link from Meteoritical Database]. In this image, obtained with an electron microprobe, the square and diamond-shaped, purplish grains are spinel, an aluminum-rich mineral. The light-green and green areas are crystals of minerals rich in both calcium and aluminum. Red area is the surrounding rock, which consists mostly of minerals rich in magnesium and iron. CAIs are thought to be among the very first solids to form in the solar nebula. Most have clear evidence for the presence of aluminum-26, but some have almost none. The variation means either that some formed millions of years after the others, or that aluminum-26 was not distributed uniformly in the solar nebula. |
The idea that aluminum-26 was not uniformly distributed in the solar nebula was extended to other short-lived, extinct isotopes as well, but it has never been proved--or disproved. Which is it? The answer is important to understanding the formation of the elements, timing of events in the solar nebula, and the overall structure and chemistry of the nebula. Nichols approached the problem by searching for isotopic effects in other elements. The formation of short-lived radioactive elements is not done in isolation--aluminum-26 here, manganese-53 there, etc. Isotopes of other elements are also manufactured. So, if aluminum-26 is distributed heterogeneously, some other isotopes will be as well. Nichols tested whether there were unusual isotopic ratios in oxygen, magnesium, silicon, potassium, calcium, titanium, chromium, iron, and nickel.
Testing the Heterogeneous-Distribution Hypothesis
The test required Nichols and coworkers to calculate the amount of isotopic variations expected to be found if supernova stuff was not mixed in homogeneously. They wrote a computer program to determine the effect of mixing debris from a supernova with a cloud whose composition matched the composition of the Sun (as determined from meteorite analyses). The composition of the supernova has been calculated by astrophysicists on the basis of nuclear physics measurements and theory. The results show that the more aluminum-26 there is in the mixture, the larger the amount of deviation of some other isotopes. The isotopes of potassium and nickel would be very distinctive, but measurable effects in the isotopes of other elements would also occur. Measurements on CAIs and other meteoritic materials, however, do not show any irregularities in isotopes. Nichols and his colleagues conclude that heterogeneous mixing of supernova debris into the pre-solar cloud did not take place, assuming aluminum-26 formed in a supernova.
More Tests
The approach taken by Nichols and colleagues is applicable to other possible sources of short-lived isotopes. For example, some isotopes might have been synthesized in red giant stars, rather than in supernova. As more precise data become available for meteorites and more calculations are done on element formation, the tests can be refined. The important point is that Nichols has developed a tool for testing ideas for formation of the Solar System, which will help us understand star and planet formation in general. The study is also an excellent example of how the boundaries between fields of study blur. This work links studies of meteorites, astronomical observations, and astrophysical calculations. Is it astronomy, planetary science, or astrophysics? It's all of them.
|
|
[ About PSRD |
Archive |
Search |
Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |