|
|
|
|
|
|
|
 |
posted Nov. 24, 1999 Purple Salt and Tiny Drops of Water in Meteorites |
Some meteorites, especially those called carbonaceous chondrites, have been greatly affected by reaction with water on the asteroids in which they formed. These reactions, which took place during the first 10 million years of the Solar System's history, formed assorted water-bearing minerals, but nobody has found any of the water that caused the alteration. Nobody, that is, until now. Michael Zolensky and team of scientists from the Johnson Space Center in Houston and Virginia Tech (Blacksburg, Virginia) discovered strikingly purple sodium chloride (table salt) crystals in two meteorites. The salt contains tiny droplets of salt water (with some other elements dissolved in it). The salt is as old as the Solar System, so the water trapped inside the salt is also ancient. It might give us clues to the nature of the water that so pervasively altered carbonaceous chondrites and formed oceans on Europa and perhaps other icy satellites. However, how the salt got into the two meteorites and how it trapped the water remains a mystery - at least for now.
Water Water Everywhere, but No Drops
Water is very abundant in the Solar System. Earth contains vast quantities in its atmosphere, oceans, lakes, rivers, and even its surface rocks and soils. The icy satellites of the outer planets contain lots of frozen water; some might even have oceans beneath their frozen crusts. It occurs in the polar caps on Mars and in the red planet's soils. It is abundant in some types of meteorites (most of which come from asteroids), but only as a component inside minerals. It has not been found in its familiar liquid form in meteorites, until now.
Water is especially abundant in carbon-bearing meteorites called carbonaceous chondrites. These meteorites have been pervasively affected by hot water flowing throughout the asteroids in which they formed. The water reacted to form a hideously complex array of minerals. Because they lack direct samples of the water that caused this confusing mess, scientists have tried to calculate the nature of the water from the nature of the products. Tough job and a very uncertain business. It would be nice to have actual samples of the water itself, and now we might.
The water might also be informative about the composition of water in the icy satellites of Jupiter, Saturn, Uranus, and Neptune. This water reacted with the rocky cores of these bodies, no doubt forming a complex array of products as on carbonaceous asteroids, so perhaps the water in the two meteorites Zolensky and his colleagues studied will shed light on this important problem, too. Water is also essential for life, so the more we know about its occurrence and distribution, the better the chance of figuring out how life took hold and where it might occur besides Earth.
Fresh Meteorites
The first meteorite found to contain salt crystals smacked into a street in Monahans, Texas, at about 7 p.m. on March 22, 1998 [Data link from Meteoritical Database]. Seven boys playing basketball witnessed the fall, which landed about 25 meters from them. They brought it to the police station soon after it fell. A Ward County Deputy Sheriff found another stone the next day, embedded in a road about 250 meters from the first one. The fall was reported on television and in newspapers (see articles from the Pecos Enterprise listed below), and when Everett Gibson of the Johnson Space Center heard about it, he immediately set out for Monahans, a small town about 30 miles west of Odessa in West Texas. Everett is from West Texas himself, from the town of Seagraves, located north of Odessa, the site of an ancient impact crater. In fact, a meteorite was found in his hometown and others were found in Brownfield to the north of Seagraves and Seminole to the south. He arrived in Monahans around noon on March 24, and brought the first piece back to Houston the same day. Thus, the sample was inside a clean, dry laboratory within 48 hours of its fall. This minimized any chance for contamination or weathering of the specimen.
People sometimes argue over ownership of meteorites. Because the rocks landed on public streets, Monahans officials claimed that both rocks belonged to the city. At the time of the discovery, Gibson promised that they would be returned to the city. However, a dispute soon developed, and the seven boys claimed ownership of the specimen that disrupted their basketball game. The city finally relented, and kept the second piece to put on display. After NASA returned the first piece to the boys, the meteorite was auctioned on the Internet (by Naturalhistoryauction.com) in July 1998. A businessman bought it for $23,000.
1998 was a good year for salty meteorites. The Zag meteorite fell on either August 4 or 5, 1998 in Morocco, in the dry Sahara desert [Data link from Meteoritical Database]. Fossil hunters witnessed its fall, and found it shortly afterwards. It was a large, 175 kg meteorite, that broke into pieces when it fell. The meteorite was apparently acquired by a dealer shortly thereafter. It seems to be available for sale by several meteorite dealers and samples have been obtained by museums, as well as by Mike Zolensky at the Johnson Space Center.
Salty Samples of Asteroid Surfaces
Monahans and Zag are ordinary chondrite meteorites that contain rock fragments in a fine-grain, dark matrix that is enriched in gases from the Sun. This means that they formed by compaction of the surface of an asteroid, which had been churned and fragmented by repeated impacts. Such meteorites, called regolith breccias, have been very informative about the nature of the interiors of asteroids and the history of the Sun. However, nobody had ever found salt in one of them.
When the sample of Monahans was cracked open in a clean lab at the Johnson Space Center, they saw purple areas up to 3 millimeters across. Closer study showed that the purple mineral was sodium chloride (NaCl), the same stuff we use for table salt. The decorative purple color (some is blue rather than purple) is due to exposure to cosmic rays while the meteorite was in space, and perhaps to radiation from the decay of radioactive potassium-40 in small grains of sylvite (potassium chloride, KCl) included in the sodium chloride. The presence of KCl inside NaCl is common in terrestrial salt deposits formed by evaporation of sea water.
  |
| Purple and blue sodium chloride in the Monahans meteorite. Each image is 1 millimeter across. |
There is nothing special about Monahans and Zag, except for the presence of salt. This suggests that other chondrite regolith breccias and perhaps other types of chondrites also contain salt. The usual procedures used to prepare samples for microscopic study use water, which would dissolve the salt. Meteorites that have been found after laying around for even a few months have gotten wet, leading to a loss of salt if it were present. Monahans and Zag were collected soon after they fell and kept dry until busted open.
Old Salt
Zolensky and his colleagues dissolved one milligram of salt from Monahans to extract the rubidium and strontium for age dating. This technique uses the fact that rubidium-87 decays into strontium-87. The data indicate that the salt in Monahans is the same age as other chondrites, 4.56 billion years. Thus, the salt formed very early in the history of the asteroid in which the Monahans meteorite formed.
Salty Water
Robert Bodner is a whiz at studying tiny fluid inclusions in minerals. This is a technical challenge and a bit of an art, and few geologists have mastered it. Not only are the inclusions small, but sample preparation can generate bogus fluid inclusions. Everett Gibson knows this all too well. In 1983, he was co-author on a study of fluid inclusions in several types of stony meteorites. The paper raised quite a stir among meteorite specialists. Unfortunately, two years later Gibson and his colleagues showed that the inclusions were artifacts of sample preparation. They suggested that the fluid used to cool the saw during sample preparation was sucked into microscopic vacuum cavities in minerals.
Bodner looked at samples of the salt from both Monahans and Zag and found small inclusions throughout them. Some have a vapor bubble inside as well. Because of great care taken during sample preparation, there is no concern about the inclusions being artifacts.
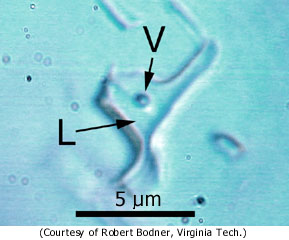 |
Photograph of a fluid inclusion in the Monahans halite. V indicates a small bubble (composed of H2O); L indicates liquid salty water. Bubbles are rare in the inclusions, indicating a formation temperature of the fluids of less than 100 degrees Celsius. |
What It May Mean
The discovery of primary salt crystals inside stony meteorites may explain a mystery about the chlorine contents of chondritic meteorites. Chondrites have elemental abundances almost identical to those of the Sun, but chlorine has always been deviant, mostly because analyses of chondrites vary widely. Perhaps that is because sodium chloride contains most of the chlorine in the rock, and it is dissolved as the meteorites sit around on Earth.
The salt was deposited after the Monahans and Zag asteroid had already formed, heated, cooled substantially, and its surface reworked by impacts. Water migrated through the ground-up surface materials, and where it evaporated, it deposited salt crystals. The salty water may have the same composition as the water that altered carbonaceous chondrites and makes up the icy satellites of the outer planets, although more detailed analysis is needed to prove that.
Where did the water come from? One possibility is that it percolated up from the interior of the asteroid in which Monahans and Zag formed. Some ordinary chondrites show evidence for alteration by hot water, so water was certainly present. Most chondrites, however, were heated to a temperature high enough that the alteration products would have been destroyed. Alternatively, the water could have come from impacts of water-bearing asteroids or comets. The impacts would release water, which would percolate throughout the piles of impact debris, forming salty deposits. It is surprising, though, that in either case no other water-bearing products, such as clay minerals or hydrated iron oxides, are formed.
The story of salt and drops of water in chondrites is just beginning.
|
|
[ About PSRD |
Archive |
Search |
Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |