|
|
|
|
|
|
|
|

|
Using Aluminum-26 as a Clock for Early Solar System Events
Written by Ernst Zinner |
A Clock and a Heat Source
26Al is a radioactive isotope that decays into 26Mg, a stable isotope, with a half-life of 0.73 million years. Although this is so short that all of it has decayed billions of years ago, its presence at the beginning of the solar system has been conclusively established by the discovery of excesses of its daughter isotope 26Mg in the most primitive solar system objects. If these objects containing 26Al at the time of their formation remained relatively undisturbed (i.e., did not experience high temperatures), the decay product 26Mg was frozen in and today provides a record of the original 26Al. The ratio of 26Mg excess measured now relative to the amount of the stable isotope 27Al yields the original 26Al/27Al ratio.
 |
|
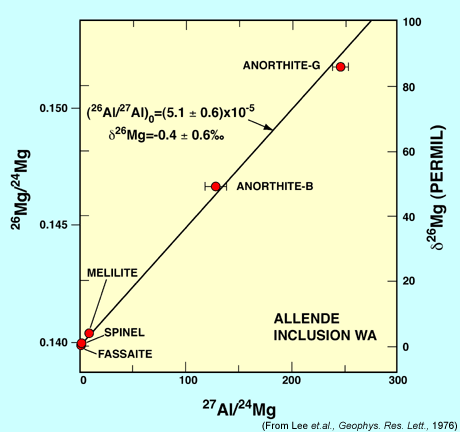
Magnesium isotopic ratios measured in different minerals with different ratios of aluminum to magnesium from a refractory inclusion in the meteorite Allende [Data link]. Magnesium shows excesses in the isotope 26 that are correlated with the aluminum/magnesium ratio, indicating that the 26Mg excesses originated from the decay of the radioactive isotope 26Al. This finding is evidence for the initial presence of 26Al in early solar system objects. |
The discovery of evidence for 26Al in the 1970s offered two very exciting prospects. The first was that this isotope could be used as a clock. The reason is that because of its radioactive decay, the 26Al/27Al ratio varies in objects that formed at different times. By measuring the aluminum-magnesium system today, the relative ages of these objects can be established. The second was that the radioactive decay of 26Al produces heat and this heat could have melted small asteroidal bodies. We have evidence for the melting of such bodies from certain types of meteorites that were produced from magmas. However, for 26Al to serve as a clock and as a heat source, two conditions had to be satisfied. The 26Al had to be distributed uniformly in the solar system (otherwise different 26Al/27Al ratios cannot be uniquely interpreted as being due to a time difference) and enough of it had to be present to provide the heat necessary for melting.
Was 26Al Uniformly Distributed?
Measurements in refractory Ca-Al-rich inclusions (CAIs) from primitive meteorites established an initial 26Al/27Al ratio of 5x10-5. This would have been enough for asteroidal melting as long as 26Al was uniformly distributed throughout the solar system and not concentrated only in CAIs and as long as small asteroids formed within a couple of million years after CAIs. It was assumed that 26Al, together with other short-lived radioisotopes, had been produced by nuclear processes (nucleosynthesis) in stars prior to the collapse of the nebular cloud giving birth to our solar system. Other primitive objects from meteorites such as chondrules show initial 26Al/27Al ratios of approximately 10-5 and smaller. This has generally been interpreted as indicating that chondrules formed approximately 2 million years after CAIs. However, it could also have meant that chondrules formed at the same time as CAIs but were endowed with less 26Al. Thus, nagging doubts remained whether 26Al was uniformly distributed. These nagging doubts were amplified by the recent discovery by Kevin McKeegan (University of California, Los Angeles) and colleagues that another short-lived isotope, beryllium-10 (half-life 1.5 million years) was also originally present in CAIs. This radioisotope is not produced by stellar nucleosynthesis but most likely formed as energetic particles from the early sun bombarded material in the accretion disk. This bombardment could, in principle, also have produced other short-lived isotopes including 26Al. If this happened mostly in CAIs, as was proposed by Frank Shu (University of California, Berkeley) and collaborators, a uniform distribution of 26Al was not assured.
Feldpars from H4 Chondrites to the Rescue
One way to establish whether 26Al can be used as a clock was to compare it to a different radioactive clock where a uniform distribution in the solar system is not in doubt. Such a clock is uranium whose isotopes 235U (half-life 0.7 billion years) and 238U (half-life 4.5 billion years) decay into lead isotopes. One fundamental difference with respect to 26Al is that the uranium half-lives are long enough that these isotopes are still around today. As a consequence, absolute ages can be measured by the uranium clock, while only relative ages can be determined with the 26Al clock. Furthermore, uranium is the only clock based on long-lived isotopes that has a precision (less than a million years) that allows the resolution of different events in the early solar system. Because lead isotopes are the daughter products of uranium decay, uranium ages are usually called Pb-Pb ages.
 Onion shell model of the parent asteroid of ordinary chondrites of type H. |
With my collaborators Christa Göpel (Laboratoire Géochimie et Cosmochimie, Paris, France) and Peter Hoppe (Max-Planck-Institut für Chemie, Mainz, Germany) I selected feldspar grains from two ordinary chondrites of type H4, Ste. Marguerite [Data link] and Forest Vale [Data link], for such a comparative study. There were several reasons for the selection of H4 chondrites. H chondrites are believed to come from a parent body that was heated (presumably by the decay of 26Al). This heating was the cause of metamorphic changes in the rocks making up this asteroid. Rocks from different depths experienced different peak temperatures and duration of heating. Correspondingly, H chondrites exhibiting different metamorphic grades are assumed to come from different depths in this parent body.
Another reason was that Christa Göpel had previously used the uranium clock on phosphate crystals from Ste. Marguerite and Forest Vale and had obtained absolute ages of 4562.7±0.6 and 4560.9±0.7 million years. These ages are so-called metamorphic ages because phosphates are metamorphic minerals that formed during heating of the H4 region on the parent body. The uranium clock thus measures a time when the temperature became low enough that the uranium and lead isotopes did not equilibrate any more with their surroundings. Compared to a uranium age of 4567.2±0.6 million years for CAIs, the time differences given by these ages are such that we could expect to find evidence for initial 26Al in H4 chondrites provided that they contain phases with a very high aluminum to magnesium ratio. This is because the 26Mg excess from 26Al decay is proportional to this ratio. Fortunately, the two H4 chondrites of our study contain fairly large (up to 0.2 millimeter) feldspar crystals with aluminum/magnesium ratios exceeding 10,000. |
 |
| Models of the temperature profiles experienced by different metamorphic grades of the H ordinary chondrites. The temperature at which the uranium-lead system stops being equilibrated during decreasing temperature is believed to be approximately 730 degrees Kelvin. This temperature is reached at different times for different H chondrites. |
Ion Microprobe Measurements of Initial 26Al/27Al Ratios
 |
This picture shows the recently installed NanoSIMS at Washington University. The NanoSIMS is a new type of ion microprobe that allows elemental and isotopic analysis with very high spatial resolution and with high sensitivity. Peter Hoppe and I measured the magnesium isotopic ratios and the aluminum/magnesium ratios in many different spots on a single feldspar crystal with such an instrument at the Max-Planck-Institute for Chemistry in Mainz, Germany. |
 |
Ion microprobe measurements of the aluminum-magnesium system in feldspar crystals from the H4 chondrites Ste. Marguerite and Forest Vale show 26Mg excesses that are correlated with the aluminum-magnesium ratio in both meteorites. The slopes of the correlation lines yield initial 26Al/27Al ratios. |
We measured the ratios of all three stable magnesium isotopes (24Mg, 25Mg, and 26Mg) and 27Al (the only stable isotope of aluminum) in several crystals from the two meteorites. On a large crystal from Forest Vale we could make these measurements in many different areas. Measurements are made by changing the magnetic field of the mass spectrometer to different values so that only ions of a given isotope are transmitted and counted. This is done through many cycles. Because of the very low magnesium concentrations, measurements take up to 10 hours for a single spot. Comparison with the magnesium isotopic ratios in terrestrial rocks revealed clear 26Mg excesses in the feldspar grains from both meteorites. The inferred initial 26Al/27Al ratios obtained from these measurements are (2.87±0.64)x10-7 for Ste. Marguerite and (1.55±0.32)x10-7 for Forest Vale.
26Al and Uranium Age Differences Between CAIs and H4 Chondrites Agree
If we interpret the differences between the widespread ("canonical") initial 26Al/27Al ratio of 5x10-5for CAIs and the ratios determined for the H4 chondrites of the present study as being due to a time difference, then we obtain for the 26Al ages of these meteorites relative to CAIs 5.4±0.1 million years for Ste Marguerite and 6.1±0.1 million years for Forest Vale. This compares to differences of 4.5±0.9 and 6.3±0.9 million years, respectively, obtained with the uranium clock. The ages obtained by the two methods are in excellent agreement.
 |
|
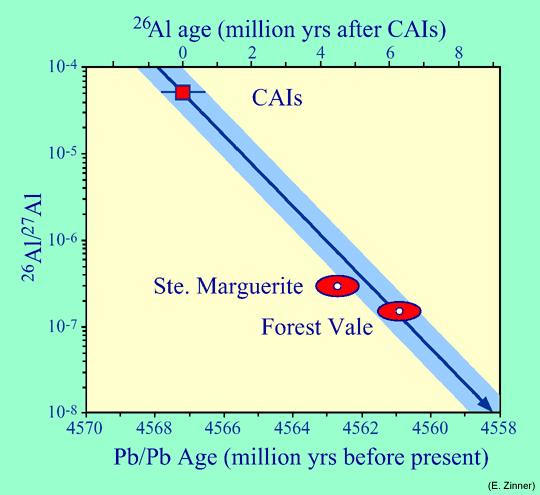
26Al/27Al ratios measured in CAIs and in Ste. Marguerite and Forest Vale as well as the ages of these objects determined with the uranium clock (Pb-Pb ages). The lower scale indicates the absolute ages, the upper scale ages relative to CAIs. The line with the arrow indicates the decrease of the 26Al/27Al ratio because of the decay of 26Al, the blue area around this line is due to the uncertainty in the absolute age of CAIs. The ellipses around the data points for the two H4 chondrites express the uncertainties of their uranium ages and 26Al/26Al ratios. Within these uncertainties the difference in the ages between CAIs and the two H4 chondrites measured by the uranium and inferred from the 26Al clock agree. |
Remaining Questions
From our analysis we have obtained an affirmative answer to our original question whether or not 26Al can be used as a fine-scale clock for early solar system events. However there are several remaining questions.
 |
|
Ages of different objects from the early solar system determined with different clocks. Only the uranium (Pb-Pb) clock gives absolute ages. The other chronometers have to be anchored to the uranium clock by measuring both systems in the same object or a set of objects. For the manganese-chromium (Mn-Cr) clock that has been done on a type of meteorite called angrites, for the iodine-xenon (I-Xe) clock the age calibration has been made on the meteorite Acapulco. For the 26Al clock we assigned absolute ages by anchoring the relative 26Al ages to the uranium age of CAIs. As can be seen, while the 26Al ages of chondrules, Ste. Marguerite (SM) and Forest Vale (FV) agree well with their uranium ages, this is not the case for the other clocks based on short-lived isotopes. For example, the 53Mn ages of Ste. Marguerite, chondrules, and especially CAIs are much older than their uranium (Pb-Pb) ages. These inconsistencies are still not understood. |
Supporting Evidence
Two recent experimental findings support our tentative conclusion that 26Al can indeed be used as a chronometer. First, Amelin (Royal Ontario Museum) and coworkers used the uranium clock to determine the absolute ages of CAIs and chondrules. [See PSRD article Dating the Earliest Solids in our Solar System.] According to their measurements, CAIs are 2.5 million years older than chondrules. This is in good agreement with the relative age difference inferred from the 26Al chronometer. Second, Marhas (Physical Research Lab, India) and colleagues reported ion microprobe measurements in unusual refractory inclusions that show the initial presence of beryllium-10 (10Be) but lack any evidence of 26Al. This indicates that 26Al was not produced together with 10Be by irradiation with energetic particles in the early solar system and removes a constraint on its uniform distribution.
While the detailed chronology of early solar system events is still far from being consistently established, our and other recent experimental studies indicate that 26Al is after all an important clock. We hope that its further usefulness can be shown in future studies.
|
|
[ About PSRD |
Archive |
Search |
Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |