|
|
|
|
|
|
|
Two Views of the Moon's Composition--- There is a striking dichotomy in estimates of the abundance of refractory elements in the Moon.
Written by G. Jeffrey Taylor |
 |
Estimates of the chemical composition of the bulk (entire) Moon fall into two drastically different categories. One group of estimates claims that the Moon is enriched in refractory elements (those that boil at high temperatures, such as calcium and aluminum) by about 50% compared to Earth. The other group claims that the abundances of refractory elements are the same in the Earth and Moon. Two papers in the same issue of a geochemistry journal fall into those two categories. Each takes a completely different approach to the problem. In one, John Longhi (Columbia University) focuses on the genesis of basalts in the mantles of the Moon and Earth. He concludes that the total aluminum concentration cannot be higher than it is in the Earth because we should see more lunar basalts as rich in aluminum as are terrestrial basalts. The other paper, by S. Ross Taylor (Australian National University), G. Jeffrey Taylor (University of Hawaii), and L. August Taylor (University of Tennessee) focuses on a mass balance between the lunar crust and mantle and tries to match up the abundances of aluminum and thorium (another refractory element). The Taylors conclude that it is likely that the Moon is enriched by about 50% in refractory elements. The discrepancy among all the studies that have been done stems from a fundamental lack of information about the composition and compositional variations in the lunar crust and mantle.
References:
Determining the bulk chemical compositions of the Moon and planets is basic to understanding how planets formed. The planet formation process involved large impacts and extensive melting, an inherently disorderly process. Nevertheless, cosmochemists and planetary physicists, optimists that they are, diligently try to figure out what the planets are made of and how they were made. See PSRD article: Gamma Rays, Meteorites, Lunar Samples, and the Composition of the Moon for more details about formation of the inner planets and the Moon, and why cosmochemists are so interested in planet compositions.
Many estimates have been made of the lunar composition. They are based on various combinations of rock compositions, experiments to simulate conditions inside the Moon, seismology, and other geophysical considerations. One of the startling things about these studies is that the estimated abundance of refractory elements fall into two distinct categories, as shown in the diagram below. One category suggests that the Moon has the same abundance of refractory elements (such as aluminum oxide, as shown in the diagram) as does the Earth (hence the Moon/Earth concentration ratio is close to 1). John Longhi's estimate falls into that group. The other category suggests that the Moon is enriched in refractory elements (Moon/Earth is 1.5 or greater). The Taylor paper (hereafter referred to as "Taylor-cubed") falls into the enrichment group. Longhi's paper and Taylor-cubed provide an interesting comparison in how different scientists address the problem of the Moon's bulk composition. The stark disagreement in the answers probably reflects our lack of sufficiently detailed information about the Moon, rather than weaknesses in the approaches taken.
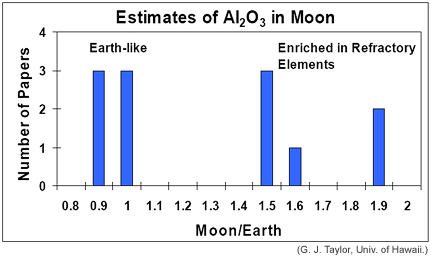 |
| The presence of a large, aluminum-rich crust on the Moon led cosmochemists to suggest that the bulk Moon was enriched in refractory elements compared to Earth. Many studies based on sample analysis, remote sensing, and geophysical studies support that idea, suggesting that the Moon is enriched in refractory elements (such as aluminum oxide, Al2O3, shown in the diagram) compared to Earth. However, other investigators suggest on equally valid grounds that the Moon is not enriched in refractory elements compared to Earth. |
John Longhi has pondered magma formation in the Moon for a long time, basing his work on careful experiments at high pressure and temperature, computer calculations based on the results of those experiments, and a vast knowledge of phase equilibria (what happens when rocks melt and crystallize). One of his interests has been the origin of the magma that produced the Apollo 15 green glass, a volcanic deposit. The green glass is what cosmochemists call "primitive," which means that it was not altered as it migrated from deep in the Moon to the surface. Thus, it reflects the composition of the lunar interior in the region where it formed.
 |
| Spherules of green glass (most about 0.1 millimeter across) collected during the Apollo 15 mission. The spherules formed by fire fountaining, a type of volcanic eruption in which escaping gases disrupt the magma into countless droplets of magma (see PSRD article: Explosive Volcanic Eruptions on the Moon). The Apollo 15 green glass magma was modified only slightly if at all from the region of the lunar mantle in which it formed, so its composition provides a window to at least some parts of the lunar interior. |
In his paper, Longhi focuses on the composition of the region of the lunar mantle that melted to produce the green glass magma. Cosmochemists call places where magma forms "source regions." The source region for the green glass magma must have been low in Al2O3 (between 1.2 and 2.4 wt%); otherwise, Longhi notes, the green glass magma would contain much more Al2O3 than it does and its crystallized form would have much more feldspar than experiments show it would have. The low Al2O3 concentration in the green glass source shows that this region did not have a composition like that of Earth's mantle, which contains about 4 wt% Al2O3, let alone a composition enriched in refractory elements (about 6 wt% Al2O3). This shows that the Moon must have melted substantially when it formed, as depicted by the magma ocean hypothesis. The green glass source contains less Al2O3 than the bulk Moon has because so much of it was concentrated in the feldspar-rich crust.
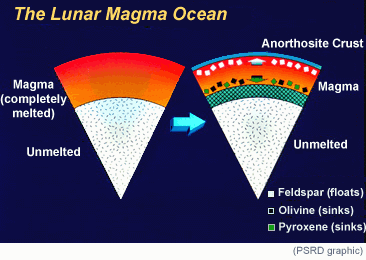 |
| There is strong evidence that an ocean of magma surrounded the Moon when it formed. As the magma crystallized, dense minerals sank and eventually plagioclase feldspar (rich in Al2O3) floated to form the primary lunar crust. The inside of the Moon ended up depleted in Al2O3. The depth of the magma ocean is not known with certainty. It was likely at least 500 kilometers thick, but John Longhi makes a good case for it being up to 1000 kilometers thick. |
Using equations based on abundant experimental data on mineral and magma compositions as a function of temperature and pressure, Longhi calculates that the green glass magma likely formed over a range of pressures as a parcel of the lunar mantle rose. This is called polybaric melting. The calculations suggest that the green glass source region began melting at a depth of between 700 and 1000 kilometers. Because the source is depleted in Al2O3, Longhi concludes that the magma ocean must have been at least that deep. Another important product from these calculations is a solid estimate of the composition of the green glass source region. Longhi gives two possibilities, which he calls GGSa and GGSp. Both estimates involve small amounts of melting at progressively decreasing pressures, with accumulation of all the melts produced. The starting compositions were varied until the accumulated magma composition matched the composition of the green glass magma. GGSa is rich in olivine and GGSp is rich in pyroxene, reasonable bounds on the green glass source region.
So, the crust has a high concentration of Al2O3 and the mantle has a small concentration. How much does that average out to? Part of the answer is "Anything you want it to," because of the numerous uncertainties in adding up the contributions from different sources. John Longhi uses a mass balance calculation, summarized in the informative but intimidating diagram below. John calculates the total amount of Al2O3 in the crust (the product of the weight fraction of crust--related to crustal thickness--and the concentration of Al2O3) and the total amount in the mantle. We know the crustal concentration reasonably well from remote sensing data, and John estimates that it is between 26 and 30 wt% (almost the same as Taylor-cubed comes up with). The thickness is less certain, so Longhi does the calculation for crustal thicknesses of 50, 60, and 70 kilometers.
Figuring out the Al2O3 concentration in the mantle is trickier. Longhi considers three cases for possible bulk Moon concentrations of Al2O3: Ross Taylor's estimate (TWM, for Taylor Whole Moon), Longhi's own estimate (LPUM, for lunar primitive upper mantle), and a chondrite composition (C). For each he considers two cases to determine the mantle composition: one where the entire Moon melted and the other where only the upper 500 kilometers melted. The importance of these two cases is that the crust is extracted from the bulk lunar silicate portion in a magma ocean. If the ocean is only 500 km deep, the resulting mantle contains less Al2O3 than in the case of whole-Moon melting. The crustal thickness matters, of course. The thicker the crust, the less Al2O3 can end up in the mantle. He compares the calculations to his estimates of the composition of the green glass source region in the mantle, assuming that it is representative of the whole mantle.
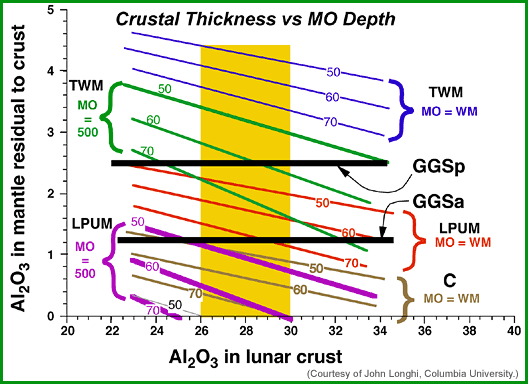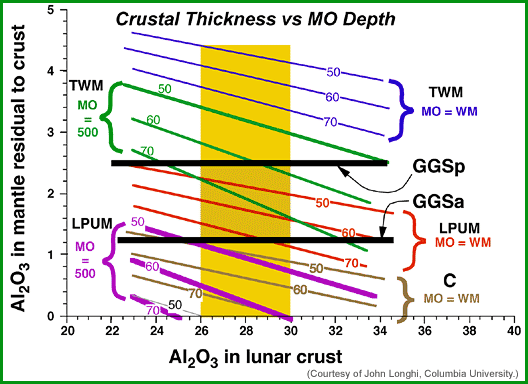 |
| Results of John Longhi's mass-balance calculations. The golden band in the center represents the range of Al2O3 in the lunar crust. The two horizontal, thick black lines represent a likely range in the composition of the source region for the green glass magma. If those source regions are representative of the whole mantle, anything falling inside the golden field between the black lines fits the constraints and could represent the bulk Moon Al2O3. The other lines represent Ross Taylor's bulk Moon estimate (TWM) for the case (blue lines) of whole-Moon melting (MO=WM) and for the case (green lines) of a magma ocean only 500 kilometers thick (MO=500). In each case the numbers on the lines represent the thickness of the lunar crust. The red and purple lines represent the same type of calculation, but for John Longhi's estimate of the lunar composition, LPUM, for whole-moon melting (red lines) and for a magma ocean only 500 kilometers thick (purple lines). The LPUM composition fills the bill no matter what the thickness of the crust is for the case of whole-Moon melting, although not for a 500-kilometer-thick magma ocean. The TWM composition seems to work only for a thick crust. A chondritic composition (C, brown lines) barely works for a thin crust and total-Moon melting, and not at all for a 500-kilometer magma ocean. |
The best estimates are those that cross the field of crustal Al2O3 between the two estimates for the green glass source region (GGSp and GGSa in the diagram). Longhi's LPUM, with a magma ocean encompassing the whole Moon (MO=WM) or one restricted to the upper 500 kilometers (MO=500) fills the bill nicely, with lots of the lines crossing the crustal field and between GGSa and GGSp. The Taylor whole-Moon composition (TWM) does not appear to work for whole-Moon melting, but can for a thick crust and shallow magma ocean. Because John Longhi's calculations indicate that the green glass source region began to melt at >700 kilometers deep, he believes that it is likely that the Moon melted almost completely, so the TWM composition is too high in Al2O3. The chondritic starting composition contains too little Al2O3 to produce the green glass source region except for whole-Moon melting, but even then it requires a mantle with less Al2O3 than the GGSa composition.
Is the case closed? The Moon is not enriched in refractory elements compared to Earth? Not necessarily!
Ross Taylor was the first to propose that the Moon was enriched in refractory elements compared to Earth, though many others have followed suit. The Taylor-cubed paper takes a new look at the idea, using data from lunar meteorites and remote sensing. We focus on the concentrations of Al2O3 and thorium. Both are refractory elements, but behave differently geochemically: Aluminum goes into pyroxene to some extent and is a major constituent in feldspar. Thorium (Th) does not concentrate in any major minerals, so it continuously builds up in a crystallizing magma. Refractory elements have the same relative abundances in all chondritic meteorites and they do not separate from each other during the condensation of a gas with the composition of the Sun. This means that if you know the abundance of one refractory element you know them all, more or less. Thus, we can constrain the Al2O3 in the mantle from the Th concentration in the crust and estimates for the mantle.
One of the hard parts of the problem is to estimate Th in the crust. We have excellent remote sensing data from the Lunar Prospector mission, but the Th concentration varies widely around the Moon. It is concentrated in the region around Oceanus Procellarum, which Brad Jolliff (Washington University in St. Louis) and his colleagues call the Procellarum KREEP terrain (see PSRD article: A New Moon for the Twenty-First Century). Th is very low in the highlands, including most of the farside. However, we do not know how the Th concentration varies with depth in the crust. Does the surface composition go all the way to the boundary with the mantle? Is there a layer beneath the surface that has much less (or more) Th? For that matter, how thick is the crust?
Taylor-cubed shows that no matter how we try, it is difficult to have the crust contain less than 26 wt% Al2O3. We found that a reasonable upper limit is 28.7 wt %. Recent interpretations of lunar seismic data suggest that the crust averages about 50 kilometers thick, which is 7.75 wt% of the bulk silicate portion of the Moon. Thus, the crust contributes 2.1 wt% Al2O3 to the total inventory on the Moon. If the mantle contains 2 wt% Al2O3 in total, the Moon would have the same total Al2O3 as does the Earth.
Like John Longhi, we used a mass balance approach to narrow down the possibilities, producing the diagram below, which is at almost as intimidating (or confusing) as is Longhi's. In it we plot Th in the crust (in parts per million, abbreviated μg/g) vs Al2O3 in the mantle. The big rectangle shaded purple indicates the range in our best estimates of Th in the crust and Al2O3 in the mantle. Superimposed are four parallel lines, each representing a different Th content in the mantle. The lines slope upwards because the total ratio of Al2O3 to Th in the bulk Moon has to be a constant (because they are both refractory elements). The horizontal dashed lines represent enrichment factors relative to Earth.
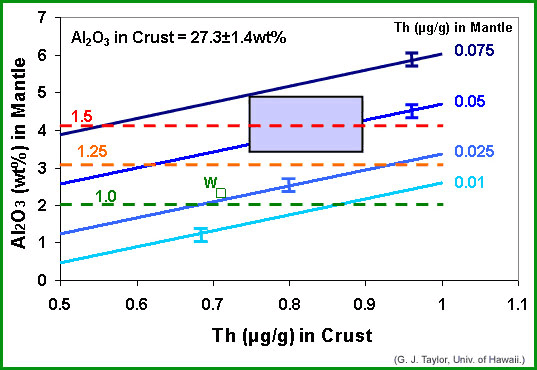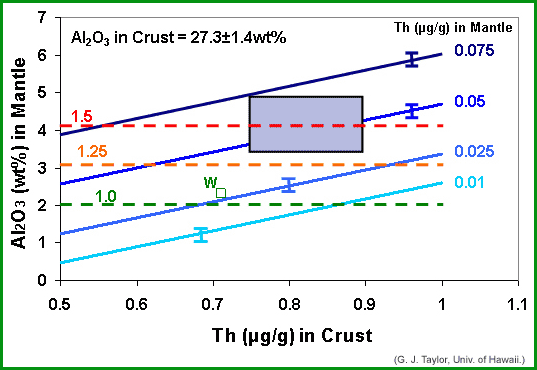 |
| Assuming that the crust is 50 kilometers thick and contains 27.3 wt% Al2O3, and the Al and Th in the bulk Moon are in the same proportions as they are in chondritic meteorites, this diagram shows the variation of Al2O3 in the mantle as a function of average Th content in the crust. Parallel solid lines show different average Th contents in the mantle. Dashed lines represent enrichment factors compared to Earth (1.0 means no enrichment; 1.5 means 50% enrichment). The purple rectangle shows the range Taylor-cubed believes are reasonable for Al2O3 in the mantle and Th in the crust. If correct, the box indicates that the Moon is enriched in refractory elements by about 50%. However, others, such as Paul Warren (UCLA) use cosmochemical data to suggest that the crust and mantle have considerably less refractories (see square labeled "W"). The biggest uncertainty is the Al2O3 content of the mantle, which Taylor-cubed base largely on geophysical data. |
Our results suggest that the Moon is at least enriched in Al2O3 and Th by 25% (above the 1.25 dashed line), and likely by 50% compared to Earth. Note, however, that there is a little box labeled with a W that is close to the 1.0 (no enrichment) line. That is Paul Warren's (UCLA) estimate (see PSRD article: Gamma Rays, Meteorites, Lunar Samples, and the Composition of the Moon for more information). Perhaps more important, our estimated enrichment of 50% suggests that the mantle has an Al2O3 concentration of almost 4 wt%. If such a source melted, John Longhi has shown that it would produce magma with much more Al2O3 than present in the green glass (or any other of the basalts that decorate the lunar maria). On the other hand, if the Al2O3 in the mantle is as low as Longhi suggests, about 2 wt%, then the crust must contain only 0.7 μg/g Th and the mantle only 0.025 μg/g. These are, in principle, testable, though we need more data to do so.
Readers might wonder how someone thinks up complicated diagrams like the two above. Mine was conceived as I was confined inside a MRI machine, getting high-tech data on why my neck hurt. I had to think about something to avoid obsessing about being trapped in a suffocating, claustrophobia-inducing diagnostic apparatus, so why not the Moon's composition? (The diagnosis, by the way, was, "You have a sore neck and there's nothing we can do about it," reaffirming my faith in modern medicine. At least no leaches were involved.)
 Depending on how we use the available data, there appears to be two schools of thought about whether the Moon is or is not enriched in refractory elements compared to Earth. But it is not just inherent biases about what we think the Moon should be made of or what we think is the most interesting answer. Several distinct approaches have been taken. The Longhi and Taylor-cubed papers show two unrelated ways of approaching the problem. There must be important insight in the different answers we get. One possibility is that the green glass source region is not typical of the lunar mantle. Perhaps it and the source regions for other mare basalt magmas constitute a small percentage of a mantle otherwise richer in Al2O3. In that case, where are the magmas rich in Al2O3 that Longhi's calculations indicate must form when such aluminous mantle melts? One answer is that they make up part of the lunar crust in the form of the suite of rocks lunar scientists call the Mg-suite. On the other hand, perhaps the Th in the crust and mantle is much lower than Taylor-cubed think, making it more like the estimate Paul Warren made.
Depending on how we use the available data, there appears to be two schools of thought about whether the Moon is or is not enriched in refractory elements compared to Earth. But it is not just inherent biases about what we think the Moon should be made of or what we think is the most interesting answer. Several distinct approaches have been taken. The Longhi and Taylor-cubed papers show two unrelated ways of approaching the problem. There must be important insight in the different answers we get. One possibility is that the green glass source region is not typical of the lunar mantle. Perhaps it and the source regions for other mare basalt magmas constitute a small percentage of a mantle otherwise richer in Al2O3. In that case, where are the magmas rich in Al2O3 that Longhi's calculations indicate must form when such aluminous mantle melts? One answer is that they make up part of the lunar crust in the form of the suite of rocks lunar scientists call the Mg-suite. On the other hand, perhaps the Th in the crust and mantle is much lower than Taylor-cubed think, making it more like the estimate Paul Warren made.
Answers are at present elusive, but might be on the horizon. At least four missions are slated to orbit the Moon during the next few years: Chandryaan (India), Selene (Japan), Lunar Reconnaissance Orbiter (USA), and Chang'e 1 (China). These missions carry an arsenal of sensors, which are bound to give us an improved view of the composition of the crust. However, the most pressing need is to probe the interior to determine the thickness and variation in thickness of the crust, and to search for compositional variations in the mantle (e.g., lower and higher Al2O3). Seismology missions might be possible with low-cost spacecraft.
|
|
[ About PSRD |
Archive |
Search |
Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |