Citation: Taylor, G. J. (April, 2011) Wet, Carbonaceous Asteroids: Altering Minerals, Changing Amino Acids. Planetary Science Research Discoveries. http://www.psrd.hawaii.edu/April11/amino_acids.html (date accessed).
 |
April 7, 2011
Wet, Carbonaceous Asteroids: Altering Minerals, Changing Amino Acids
--- Aqueous alteration in asteroids containing organic compounds leads to formation of hydrous minerals and changes in the mix of amino acids.
Written by G. Jeffrey Taylor
Hawai'i Institute of Geophysics and Planetology
Many carbonaceous chondrites contain alteration products from water-rock interactions at low temperature and organic compounds. A fascinating fact known for decades is the presence in some of them of an assortment of organic compounds, including amino acids, sometimes called the building blocks of life. Murchison and other CM carbonaceous chondrites contain hundreds of amino acids. Early measurements indicated that the amino acids in carbonaceous chondrites had equal proportions of l- and d-structures, a situation called racemic. This was in sharp contrast to life on Earth, which heavily favors l- forms. However, beginning in 1997, John Cronin and Sandra Pizzarello (Arizona State University) found l- excesses in isovaline and several other amino acids in the Murchison carbonaceous chondrite. In 2009, Daniel Glavin and Jason Dworkin (Astrobiology Analytical Lab, Goddard Space Flight Center) reported the first independent confirmation of l-isovaline excesses in Murchison using a different analytical technique than employed by Cronin and Pizzarello.
Inspired by this work, Daniel Glavin, Michael Callahan, Jason Dworkin, and Jamie Elsila (Astrobiology Analytical Lab, Goddard Space Flight Center), have done an extensive study of the abundance and symmetry of amino acids in carbonaceous chondrites that experienced a range of alteration by water in their parent asteroids. The results show that amino acids are more abundant in the less altered meteorites, implying that aqueous processing changes the mix of amino acids. They also confirmed the enrichment in l-structures of some amino acids, especially isovaline, confirming earlier work. The authors suggest that aqueously-altered planetesimals might have seeded the early Earth with nonracemic amino acids, perhaps explaining why life from microorganisms to people use only l- forms to make proteins. The initial imbalance caused by non-biologic processes in wet asteroids might have been amplified by life on Earth. Alternatively, the same processes that produced the l-amino acid excesses in carbonaceous asteroids also operated on the early Earth.
Reference:
- Glavin, D. P., Callahan, M. P., Dworkin, J. P., and Elsila, J. E. (2011) The Effects of Parent Body Processes on Amino Acids in Carbonaceous Chondrites. Meteoritics and Planetary Science, v. 45(12), p. 1948-1972, doi: 10.1111/j.1945-5100.2010.01132.x
- PSRDpresents: Wet, Carbonaceous Asteroids: Altering Minerals, Changing Amino Acids --Short Slide Summary (with accompanying notes).
Wet and Gunky Meteorites
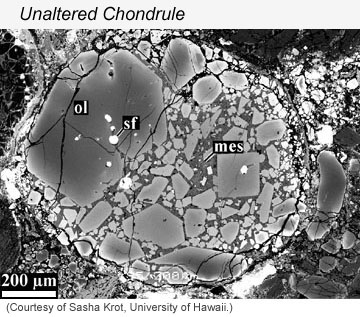
Carbonaceous chondrites have a range of properties. Some are wetter than others, some have more carbon than others, some have much higher concentrations of organic compounds than others. Daniel Glavin and his co-authors focused on those with significant amounts of organic compounds--up to only 2-3%, but that's enough to give the meteorites a distinctive earthy smell, a bit like tar. Their set of meteorites also included a range in the amount to which water affected their primary anhydrous minerals to produce a host of complicated hydrous minerals. Examples of the effects of aqueous alteration are shown in the electron microscope images below.
 |
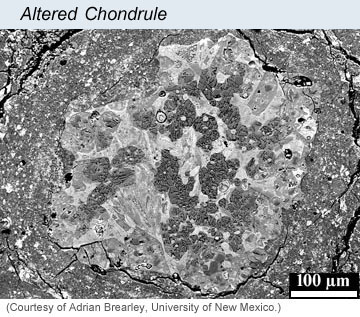 |
| [Left] Image taken in a scanning electron microscope using backscattered electrons of a typical chondrule in a meteorite that has not experienced aqueous alteration. The chondrule, which fills the field of view, is composed mostly of olivine (ol) with inclusions of iron sulfide (sf). None of the olivine crystals are altered. Even the mesostasis (mes), the last of the molten chondrule to crystallize, is unaffected. [Right] Backscattered scanning electron micrograph of a typical chondrule (specifically what cosmochemists call a Type IAB chondrule) in the CM chondrite ALH 81002. The high-temperature primary mineral enstatite has been partially altered to serpentine (ragged, darkest gray crystals). In the outer parts of the chondrule the enstatite crystals have been almost completely replaced by Mg-serpentine, though the outlines of the enstatities are preserved. The brighter interstitial regions are mainly altered mesostasis glass that has been replaced by an early generation of Fe-serpentine. This chondrule is surrounded by a broad, fine-grained rim. | |
Chondritic meteorites vary substantially in their properties, although all but one type contain chondrules, rounded, millimeter-sized objects that were at one time molten droplets. They are further classified by the amount of alteration they experienced. For historical reasons, the most unaltered ones fall into Type 3. Thermal metamorphism caused some to be heated to several hundred degrees, producing Types 4 through 6, with Type 6 being heated to the highest temperature (about 900 oC). In chondritic asteroids that contained water (almost certainly ice to begin with), heating was less severe, but alteration was considerably more as water flowed in cracks, permeating asteroid interiors. The water reacted with the original chondrule minerals to form hydrous alteration products. Aqueously-altered chondrites are classified as Type 1 and 2, with Type 1 being the most altered, so altered in fact, that chondrules are not even visible.
Chondrites are also divided into groups based on chemical and isotopic compositions, and then named after a typical chondrite in the group (usually one that was observed to fall). For carbonaceous chondrites, the groups include CI (Ivuna-like), CM (Mighei-like), CR (Renazzo-like), CO (Ornans-like), CV (Vigarano-like), and CK (Karoonda-like). Carbonaceous chondrites also contain carbon, including organic compounds, which we discuss below. Combined with the alteration classification, meteorites are classified as, for example, CM1 or CM2, CR1 and CR2, and so on. A summary of the classification scheme appears below; colored boxes show the categories in which we have identified carbonaceous chondrites.
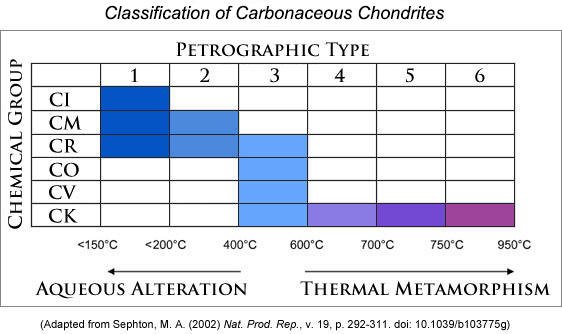 |
| Carbonaceous chondrites are classified by their bulk chemical composition, which places them into groups (CI, CM, etc.), and by the amount of aqueous alteration (petrographic types 1 and 2) and heating without much water (3 through 6). Areas in the table are colored to signify which categories have been seen. Darker blue colors represent more aqueous alteration and redder colors represent increased thermal metamorphism. |
Daniel Glavin and his co-authors studied the amino acids in carbonaceous chondrites that exhibited a range of aqueous alteration. Their goal is to understand the role aqueous alteration plays in changing amino acids (decomposing some, synthesizing others, changing their relative abundance, and possibly enhancing the ratio of l- to d-amino acids).
Amino Acids
Amino acids are the chemical building blocks that make proteins. DNA is coded to use only twenty of them in protein construction, but organisms use other amino acids for assorted biological functions. The Murchison CM2 chondrite [Data link from the Meteoritical Bulletin] contains over 100 different amino acids, only eight of which are used in the proteins in terrestrial life. Some appear to be presolar grains (PSRD article: Interstellar Organic Matter in Meteorites). The essential components of amino acids are an amine group (NH2), which contains the nitrogen, and a carboxylic acid group, COOH. These are joined to numerous types of other functional groups, called side chains, to make up a menagerie of amino acids. Amino acids can also contain extra carbon atoms, designated as, for example, α-alanine, β-alanine, etc., depending on which carbon atom the amine group is attached to.
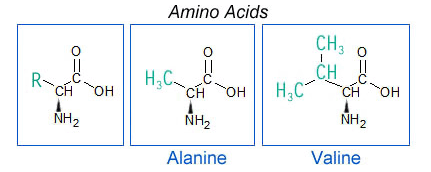 |
| Amino acids have two main components, an amine group (containing nitrogen) and a carboxylic acid group. These join with other molecular groups (called side chains) to make different amino acids, as depicted by the R in the diagram on the left. The differences in the nature of the side chains lead to different chemical compositions and uses of each amino acid, such as alanine (center) and valine (right). Most amino acids are more complicated than those shown. |
Due to the geometry of many amino acids they often come in two structural shapes, one a mirror image of the other, but with the same chemical formula (a property called chirality). Either one of the pair is called an enantiomer. These mirror images are designated l and d. Biology prefers l, and all the amino acids in proteins are in the l- configuration (designated by names such as l-alanine or l-valine).
 |
Almost all amino acids have symmetrical forms, designated l and d. The one shown in this artist's illustration is isovaline, which is one of the most abundant amino acids in carbonaceous chondrite meteorites. |
Assaying Amino Acids
The Astrobiology Analytical Lab at Goddard Space Flight Center teems with state-of-the-art instruments to measure the types of organic compounds and their abundances in almost any type of sample. For this study they used a high performance liquid chromatograph coupled to a time-of-flight mass spectrometer. These tools allow measurement of amino acids in solutions from chemically-treated samples. The use of a special detector in the chromatograph and coupling to the time-of-flight mass spectrometer vastly improves the detection limit of amino acids compared to current state-of-the-art gas chromatography-mass spectrometer measurements. This vast improvement in performance is essential because of the extremely low abundances of individual amino acids even in carbonaceous chondrites and because only limited amounts of these rare meteorites are available.
Before the high-tech gizmos can be used, the amino acids have to be extracted from the meteorite samples. This crucial procedure required use of ultra-clean glassware, ceramic containers, and sample-handling tools. All reagents and water were of the highest purity available. Extraction steps included heating, exposure to hydrochloric acid, cation-exchange resins, and chemical modification (derivatization) with o-phthaldialdehyde/N-aceteyl-L-cysteine. (The use of these toxic chemicals explains why chemistry laboratories have fume hoods and emergency showers.) Analyses indicate that even after all that treatment, the amino acids in the samples were not degraded.
As a testament to how complicated the entire wet chemical and instrumental procedure is, just glance at the wonderfully technical list of ingredients in the standards Glavin and his co-workers prepared for this research:
"Individual d- and l-2-amino-2-methylbutanoic acid (2-a-2-mba, isovaline) were purchased from Acros Organics (>99% purity), and a racemic mixture (d = l) was prepared by mixing the appropriate volumes of each individual solution. dl-2-aminopentanoic acid (2-apa, norvaline), dl-2-amino-3-methylbutanoic acid (2-a-3-mba, valine), and 5-aminopentanoic acid (5-apa) standards were purchased from Sigma-Aldrich (97-99% purity). dl-3-aminopentanoic acid (3-apa) was from AzaN (97% purity). 3-amino-3-methylbutanoic acid (3-a-3-mba), 3-amino-2,2-dimethylpropanoic acid (3-a-2,2-dmpa), dl-3-amino-2-methylbutanoic acid (3-a-2-mba and allo- 3-a-2-mba, 4 stereoisomers), dl-4-aminopentanoic acid (4-apa), dl-4-amino-3-methylbutanoic acid (4-a-3-mba), l-3-amino-2-ethylpropanoic acid (3-a-2-epa), dl-4-amino- 2-methylbutanoic acid (4-a-2-mba) were individually synthesized and provided by S. Pizzarello (ASU), S. Miller (UCSD), S. Davies (Oxford), and R. Duke (U. Sydney). The l-3-a-2-epa was mostly racemized by 6 M HCl acid vapor hydrolysis (150 _C for 1 week)."
Changes in the Amino Acid Set as Asteroid Water Flowed
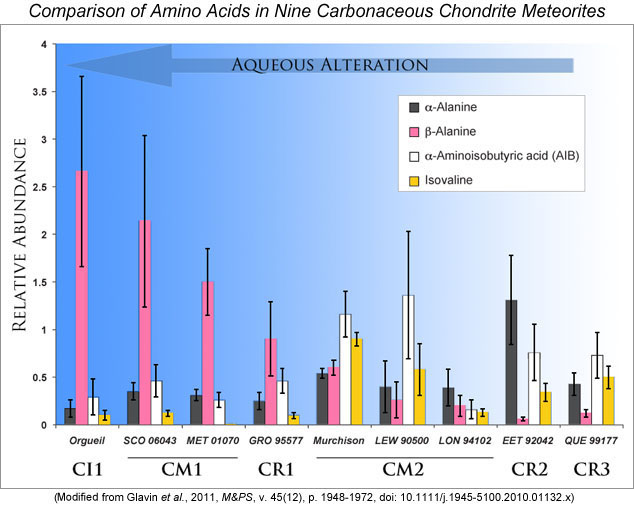
Daniel Glavin and his team present the first large database of amino acids in a broad suite of carbonaceous chondrites representing petrographic types 1 through 3, hence a range in the extent of aqueous processing, and all measured with the same experimental protocol and instruments. This allows them to take a close look at how aqueous alteration affected the primary amino acids in carbonaceous asteroids. The graph below shows their results in simplified summary form, with the degree of aqueous alteration increasing from right to left. A striking change is the increase in the relative abundance of β-alanine with more aqueous alteration. Orgueil, the most altered petrographic type 1 chondrite, has the most β-alanine, and some type 2 chondrites have no detectable β-alanine. Although it is possible that the increase in β-alanine is due to differences among the parent asteroids of the meteorites studied, Glavin and his coworkers suggest that it is more likely that the extent of aqueous processing is the key difference.
 |
| Comparison of the relative abundances of four amino acids in carbonaceous chondrites. All abundances are normalized to the amount of glycine present (which if plotted would always have a value of 1). The error bars represent the uncertainties derived from the standard deviations of 4 to 8 analyses for each meteorite. β-alanine increases with increasing amount of aqueous alteration. The abundance of isovaline appears to decrease with alteration. |
Excess of l over d Structures
Researchers had previously reported small excesses of l over d structures in amino acids in carbonaceous chondrites. Glavin and colleagues confirm these excesses (see graph below). It appears that the percentage of l-isovaline is greater in the more aqueously altered chondrites. Orgueil, SCO 06043, GRO 95577, and Murchison all have l excesses well outside experimental uncertainties (shown by the bars in the figure). In the other, less altered samples, the l excess is zero within experimental uncertainty. A concern with an l excess is that it is caused by contamination once the meteorite landed on Earth, where amino acids are almost entirely l. However, in a 2009 paper, Daniel Glavin and Jason Dworkin address the analytical and contamination issues in detail. They conclude that contamination of the interiors of the meteorite samples is unlikely; the l-isovaline excess is of extraterrestrial origin and not an analytical artifact. They also point out that the concentrations of isovaline on Earth are very small. Contamination of the other amino acids is thus more likely, and those that make up proteins have l/d of 1, indicating no contamination.
Glavin and coworkers conclude that the processes involved in aqueous alteration cause the l-isovaline excesses. Researchers had thought that the small excesses measured previously were caused by pre-accretion processes in the solar nebula, such as irradiation with ultraviolet light. While possible, the correlation between aqueous alteration and l excess indicates a role for alteration by water in the parent asteroids of carbonaceous chondrites.
 |
| This chart shows l excess [l/(d+l) isovaline, expressed as percent] for each chondrite studied, listed in decreasing amount of aqueous alteration from left to right. Experimental uncertainties are shown by the bars and are based on standard deviations of between 8 and 23 analyses for each chondrite. The four samples on the right are not enriched in l-isovaline within experimental uncertainties. The four on the left are clearly enriched. |
Organics on the Early Earth
In a paper published in 1992, Chris Chyba (now at Princeton University) and Carl Sagan suggested that asteroids similar to carbonaceous chondrites may have delivered a significant complement of organic compounds to Earth very early in its history. Following an idea by Sandra Pizzarello (Arizona State University) and Art Weber (SETI/NASA Ames Research Center), Daniel Glavin and his co-authors suggest that although not abundant, amino acids such as isovaline might have served as catalysts for organic reactions, hence transferring their asymmetry to other amino acids. Perhaps, they suggest, life on Earth was biased towards l-amino acid asymmetry from the beginning. This does not rule out the possibility that aqueous alteration of mixtures of minerals and organic compounds on Earth led to the l enrichment, just as it did in carbonaceous asteroids. Perhaps aqueous alteration led to the l-amino acid bias no matter where the alteration occurred.
- PSRDpresents:
Wet, Carbonaceous Asteroids: Altering Minerals, Changing Amino Acids --Short Slide Summary (with accompanying notes).
- Astrobiology Analytical Lab at Goddard Space Flight Center
- Chyba, C. and Sagan, C. (1992) Endogenous Production, Exogenous Delivery and Impact-Shock Synthesis of Organic Molecules: An Inventory for the Origins of Life, Nature, v. 355, p. 125-132.
- Cronin, J. R. and Pizzarello, S. (1997) Enantiomeric Excesses in Meteoritic Amino Acids, Science, v. 275, p. 951-955.
- Glavin, D. P., Callahan, M. P., Dworkin, J. P., and Elsila, J. E. (2011) The Effects of Parent Body Processes on Amino Acids in Carbonaceous Chondrites. Meteoritics and Planetary Science, v. 45(12), p. 1948-1972, doi: 10.1111/j.1945-5100.2010.01132.x
- Glavin, D. P. and Dworkin, J. P. (2009) Enrichment of the Amino Acid L-isovaline by Aqueous Alteration on CI and CM Meteorite Parent Bodies, Proceedings of the National Academy of Sciences, v. 106(14), p. 5487-5492, doi: 10.1073/pnas.0811618106.
- Pizzarello, S. and Weber, A. L. (2004) Prebiotic Amino Acids as Asymmetric Catalysts, Science, v. 303, p. 1151.
- Sephton, M. A. (2002) Organic Compounds in Carbonaceous Meteorites, Natural Product Reports, v. 19, p. 292-311, doi: 10.1039/b103775g.
- Taylor, G. J. (May 2006) Interstellar Organic Matter in Meteorites. Planetary Science Research Discoveries. http://www.psrd.hawaii.edu/May06/meteoriteOrganics.html.
|
|
[ About PSRD | Archive | CosmoSparks | Search | Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |
