Citation: Taylor, G. J. and Martel, L. M. V.(November, 2011) Festival on the Formation of the First Solids in the Solar System. Planetary Science Research Discoveries. http://www.psrd.hawaii.edu/Nov11/FormationFirstSolids.html (date accessed).
 |
November 30, 2011
Festival on the Formation of the First Solids in the Solar System
--- Cosmochemists, astronomers, and astrophysical modelers shared data and ideas about the formation of the materials making up the Solar System.
Written by G. Jeffrey Taylor and Linda M. V. Martel
Hawai'i Institute of Geophysics and Planetology
Over 180 cosmochemists, astrophysical modeling experts, and astronomers met on the island of Kaua‘i, Hawai‘i at the Workshop on Formation of the First Solids in the Solar System. The meeting was hosted by the Hawai‘i Institute of Geophysics and Planetology (HIGP), School of Ocean and Earth Science and Technology (SOEST), the NASA Astrobiology Institute, and the Institute for Astronomy (IfA), all at the University of Hawai‘i at Mānoa. Conveners were our colleagues Sasha Krot, Ed Scott, Jonathan Williams, Gary Huss, and Martin Bizzarro (Natural History Museum of Denmark), and Yuri Amelin (Australian National University).
Reference:
- Workshop on Formation of the First Solids in the Solar System (November 7—9, 2011) Full program and listing of abstracts.
About the Workshop
The stimulating and energetic workshop addressed fundamental questions about where we and everything around us came from. Central themes included:
- Whether short-lived isotopes such as aluminum-26 and stable isotopes were distributed uniformly or heterogeneously in the disk of gas and dust surrounding the proto-Sun, and the nature of possible processes that could have led to clumping of materials to cause heterogeneities.
- How chondrules and calcium-aluminum-rich inclusions (CAIs) formed and then modified, including the age-old unsolved problem of how oxidized iron wound up in chondrules.
- Which components were produced or modified in stars no longer in existence, in interstellar medium clouds before the solar system formed, and in the Solar System.
- The cause of the variation in oxygen isotopic compositions from primitive components in chondrites to entire planets.
- And the whole question of the relation among the menagerie of stable and radioactive elements (short and long half-lives) and where they formed.
Great data and ideas abound, and pressing questions remain in this vibrant area of research. We give an overview of the workshop to try to capture the essence of the galactic-scale issues discussed by this diverse gathering of people trying to figure out our origins.
 |
The meeting was dedicated to Professor Klaus Keil (University of Hawai‘i) to honor his distinguished career in meteoritics and cosmochemistry. Dr. Keil pioneered the use of the electron microprobe to determine the chemical composition of minerals in meteorites. He is one of the co-inventors of the energy-dispersive x-ray spectrometer used on electron microprobes and electron microscopes to analyze small samples. His wide-ranging research has covered all types of meteorites and Apollo lunar samples.
|
Clumps of Star Stuff?
Short-lived isotopes are important for monitoring the compositional uniformity of the proto-solar disk and for determining the timing of events early in the history of the Solar System. Short-lived isotopes have half-lives that range from days (beryllium-7) to 82 million years (plutonium-244). A particularly useful one is aluminum-26 (26Al), whose 700,000-year half-life allows it to date events during the first several million years of the Solar System. In introducing a session devoted to short-lived isotope abundances in meteorites, Martin Bizzarro (Natural History Museum, Denmark) pointed out that some talks emphasized the homogeneity in the distribution of 26Al, while others emphasized that it is distributed heterogeneously. Both answers have interesting implications. Heterogeneous distribution suggests addition to the proto-solar disk or its precursor molecular cloud by a mechanism that did not allow complete mixing. Homogeneous distribution of 26Al makes it an excellent chronometer for determining the sequence of events in the proto-solar disk.
But were 26Al and other short-lived isotopes distributed uniformly or not? The answer is ambiguous, although the evidence for at least some heterogeneity in 26Al is growing. For example, Sasha Krot (University of Hawai‘i) and coworkers showed that about half of the corundum grains in chondrites had very low abundances of 26Al when they formed. This is important because corundum (Al2O3) is one of the first solids to form in the Solar System, so it ought to have the highest 26Al abundance rather than the lowest, even as low as not having any detectable 26Al.
 |
This corundum grain (cor) is in the Murchison carbonaceous chondrite. Scale bar is only 1 micrometer. The dark oval in center is the hole made during analysis by ion microprobe. |
 |
 |
| [LEFT] Measurements of the apparent initial 26Al/27Al ratio in corundum (Al2O3) grains separated by acid leaching and of corundum grains measured in situ in ordinary and carbonaceous chondrites. Note the large peak (shown in yellow) at 26Al/27Al much lower than the value of 5.2 x 10-5 typically thought of as the initial ratio in the Solar System. This implies either that the corundum grains formed late enough that all the 26Al had decayed away (unlikely as corundum is the first mineral to form from the hot regions in the disk), or that it had not been mixed into the region of the proto-solar disk in which the corundum formed. [RIGHT] Oxygen isotopic compositions plotted against the initial 26Al/27Al ratio in corundum grains. The oxygen notation is the distance of the composition from the terrestrial line on an isotope plot of the three oxygen isotopes. The blue, horizontal band shows the composition of the Sun as measured by samples from the Genesis solar wind sample-return mission and the orange, vertical band is the composition thought to represent the initial 26Al/27Al in the Solar System, as obtained from measurements of calcium-aluminum-rich inclusions. Oxygen isotopic composition does not correlate with the initial 26Al/27Al ratio. | |
Reports supporting the notion that 26Al was not distributed uniformly in the proto-solar disk were also given by Ming-Chang Liu (CRPG-CNRS, Nancy, France) and coworkers, Kirsten Larsen (Natural History Museum of Denmark) and coworkers, and Noriko Kita (University of Wisconsin, Madison) and colleagues, and hinted at by others in their presentations and during discussions.
The heterogeneous distribution of 26Al implies that this isotope was heterogeneous in the molecular cloud from which the Solar System formed and did not become uniform until collapse into the Sun and protosolar disk was completed. Alternatively, 26Al might have been injected into the disk after it had formed. Both possibilities have their proponents. Models of Solar System formation in star-forming regions provide some support for addition of 26Al to the protosolar disk. One idea, explained by Steve Desch (Arizona State University), centers around the concept that supernova ejecta are clumpy. They spew out fingers of dense, 26Al-rich clumps of fast-moving material. The clumps enrich small portions of the surrounding molecular cloud, creating irregularities in the distribution of 26Al. A small fraction will even hit disks as they are forming, like cosmic Cupid's arrows. The 26Al-rich clumps would not have mixed instantly, leading to the possibility of heterogeneous distribution of 26Al and other isotopes produced by supernovae. Alan Boss (Carnegie Institution of Washington) showed models of the effects of numerous fingers (clumps) arriving on the surface of the still-forming proto-solar disk, leading to an initial heterogeneous distribution of 26Al.
 |
| Hubble Space Telescope image of supernova remnant Cassiopeia A. Colors show compositional variations. Note the clumping of materials, making some regions bright. Steve Desch points out that the clump size is about the size of the disk surrounding the Sun as it was forming. Models of supernova explosions also indicate formation of clumps as the exploding star expands. |
Iron-60 is another interesting short-lived isotope. It has a half-life of 2.62 million years. Like 26Al, its initial abundance has significance for the astrophysical environment in which the Solar System formed. Thus, cosmochemists are going to great lengths to determine the initial amount of 60Fe (expressed as the 60Fe /56Fe ratio) and the extent to which it was distributed uniformly. The current status seems to be that its abundance is fairly low, 60Fe /56Fe of about 1 x 10-8 and appears to be decoupled from 26Al. It is not clear that it was distributed heterogeneously or uniformly in the early Solar System, though data reported by several groups indicate a uniform initial ratio among differentiated meteorites (those that melted during the first few million years of Solar System history). Most importantly, the low inferred abundance of 60Fe could have been inherited from the molecular cloud in which the Solar System formed, with no late injection required.
Processing Nanodiamonds, GEMS, and Other Valuables
Meteorites, comet dust samples, and interplanetary dust particles (IDPs) contain stardust, presolar grains that originally formed in asymptotic giant branch (AGB) phase stars and supernovae. Once liberated from these stellar sources, the grains resided in an interstellar molecular cloud for an unknown length of time, but likely for thousands to millions of years. During this time they would have been subjected to radiation and high pressures and temperatures associated with supernovae, thereby altering their original state. At some point the grains would have been swept up into the collapsing cloud that formed the Solar System, leading to possible alteration in the proto-solar disk and eventually in asteroidal and cometary parent bodies. Looking through the haze of all these events to determine the history of each stardust grain is a major area of research, a great challenge, and a source of argument.
Many presolar grains are amorphous. They may have chemical compositions similar to minerals, but are not crystalline; they lack long-range atomic order. Did these grains ever have long-range order? If they were crystalline, what process disordered the crystal structure and where did that happen? These questions come into sharp focus when considering the origin of GEMS, an acronym for "glass with embedded metal and sulfides." John Bradley (Lawrence Livermore National Laboratory) coined the term in 1994 for the already well-documented grains found in large quantities in the type of IDPs linked to cometary sources. Typically just 0.1-0.5 micrometers in diameter, GEMS grains are made of nanometer-sized sub-grains of kamacite (FeNi metal) and Fe-Ni sulfide in a Mg-Fe-Al amorphous silicate matrix.
 |
| Bright-field transmission electron microscope image of a cluster of GEMS grains held together with carbonaceous material. |
The mega-debate about these nano-scale objects involves the interpretation of where the particles formed. Was it in a presolar environment or in the fledgling Solar System? The presolar-origin hypothesis, endorsed by John Bradley, says GEMS grains began as free-floating, crystalline mineral grains that were exposed to ionizing radiation in the interstellar medium (ISM). The radiation chemically and isotopically homogenized the grains by prolonged sputtering and redeposition before being swept into the cloud of dust and gas from which our Solar System formed. Bradley and colleagues have found embedded relict grains, such as pyrrhotite (FeS) and forsterite (MgSiO4), inside some GEMS grains, which they interpret as remnants of the original crystals from which they formed.
The solar nebula-origin hypothesis says GEMS grains are Solar System products that formed as late-stage non-equilibrium condensates. Lindsay Keller (NASA Johnson Space Center) and colleagues argue GEMS grains display enormous chemical variability, which is inconsistent with the idea of chemical homogenization. They say the observed heterogeneities point to non-equilibrium conditions during grain formation and may reflect the changing composition of the gas from which they condensed. They suggest that if there are relict core grains, they were a base for the accumulation of material either through simple aggregation or, possibly, as condensation nuclei.
If GEMS grains are presolar, then they would meet the benchmark that establishes a presolar origin: Nonsolar oxygen isotopic composition (enriched in 17O, depleted in 18O). Indeed, some GEMS grains meet this benchmark (a few percent of the total analyzed so far), but all the rest have oxygen isotopic compositions within the range of Solar System materials. While Keller and colleagues agree that a small percentage of GEMS grains are surviving presolar grains based on nonsolar oxygen isotopic compositions, they contend the overwhelming solar isotopic compositions indicate a solar nebula origin. Bradley and colleagues argue that the evidence of presolar origin was erased; most GEMS grains were so extensively irradiated, modified, and homogenized in the ISM that they no longer retain their original, nonsolar isotopic compositions.
 |
Another interesting type of presolar material are nanodiamonds, diamonds that are only a few nanometers across. These small objects are found by using strong acids to dissolve almost all of a meteorite. The acid-resistant residue contains assorted oxides, silicon carbide, and other phases, and nanodiamonds. The nanodiamonds tend to occur in clusters. Published measurements of krypton and xenon isotopes suggest a presolar origin, although abundances of carbon and nitrogen isotopes are like those in the Solar System. Thus, it is not clear that nanodiamonds are products of stellar or Solar System processes.
Rhonda Stroud (U.S. Naval Research Laboratory) and coworkers studied acid residues from the Murchison carbonaceous chondrite. They found that nanodiamonds are associated with a disordered carbon phase, which they interpret from detailed electron microscopy to be glassy carbon, a curious form of carbon that is known from laboratory experiments to be impermeable to gas flow and resistant to dissolution by acids.
[LEFT] Transmission electron microscope image of a nanodiamond separate from the Murchison meteorite. Nanodiamonds (ND) have crystalline structures as shown by the orderly internal structure. Glassy carbon (GC) is not ordered. Small bright spots are individual atoms of impurities such as iron, magnesium, fluorine, and sulfur, which are most likely contaminants from the acid dissolution process.
Experiments show that glassy carbon can transform to nanodiamond at temperatures in the range 1600 to 2000 Celsius and pressures of 15 Giga Pascals (about 150 times atmospheric pressure at Earth's surface). Stroud and colleagues suggest that the mixture of diamond and glassy carbon could have been produced by a shock wave from a supernova that transformed pre-existing organic compounds in the interstellar medium.
Tiny Droplets
 |
Chondrites get their name from the presence of countless millimeter-sized, silicate spherules that crystallized from a melt. They are 1-3 million years younger than CAIs and their origin has been the subject of intense discussion for decades. The First Solids workshop did not settle any arguments, but did shed light on some possible processes involved in the formation of these abundant and intriguing objects. We highlight some of that fresh light here.
[RIGHT] The Semarkona chondrite is composed of numerous chondrules, the round objects evident in this photograph of a thin slice of the meteorite. The chondrules are about a millimeter across. Their abundance demands that we understand how they formed.
One central characteristic of chondrules is the existence of two important types, designated types I and II. Type I contain only small amounts of oxidized iron (FeO); olivine crystals in them contain only about 2 mole percent of the fayalite (Fe2SiO4) end member. Type II chondrules contain much more FeO; olivine crystals in them typically contain 10-30 mole percent fayalite. Why are they different? More important, even the type I chondrules have more FeO than expected for condensation in a gas with the composition of the Sun, which is essentially none at high temperature, where pure forsterite, Mg2SiO4, is chemically stable. At low temperature, olivine would contain more fayalite, but the temperature is too low to allow it to move into the mineral. This has been quite a dilemma. Larry Grossman summarized the state of the problem, quantitatively examining assorted processes that might cause more FeO to condense earlier, more of it to form (e.g., by having more H2O present in the solar gas), or to move into crystals faster. The fundamental problem is that the oxidation conditions (expressed as the oxygen fugacity, roughly related to the partial pressure of available oxygen) to form FeO are 100,000 to a million times higher than existed in the solar nebula (protosolar disk). He also showed that the difference in oxygen fugacity needed to form type I chondrules is trivial, substantially less than a factor of 10 of that required to form type II chondrules.
Guy Libourel and colleagues at CRPG-CNRS (Nancy, France) reported on a series of experiments designed to test the idea that type II chondrules could be derived from type I chondrules by oxidation. An interesting feature of type II chondrules is that olivine crystals in them often have cores that resemble olivine in type I chondrules, suggesting that perhaps type II could be modified versions of type I chondrules. The idea is that metallic iron in type I chondrules could be oxidized to FeO, which would move into the olivine crystals, forming type II chondrules. Incomplete diffusion of the Fe would leave a ghost of the original olivine. Experiments at different oxidation states (oxygen fugacities) confirmed that the process is feasible. Where it happened, how long it took, how the original type I chondrules formed, and where the oxidation agent came from remain to be determined.
 |
 |
| [TOP row photomicrographs] Backscattered electron images of chondrules in the Semarkona chondrite, from type I (left) and a variety of type II chondrules with variations in the abundance of relict olivine (compositions like those in type I chondrules) in grain centers (darker than areas richer in iron). [BOTTOM row photomicrographs] Images showing the results of experiments at the Centre de Recherches Pétrographiques et Géochimiques (CRPG-CNRS) beginning with material like type I chondrules (left image) that become progressively more oxidized to the right. Oxidation conditions were the same for all experiments, a factor of ten above the oxygen fugacity where FeO and metallic iron coexist. In both sets of images, the abundance of relict (composition like those in type I chondrules) is shown by the gray prism. |
Another puzzle is why little sodium and potassium seem to have been lost from chondrules (see PSRD article: Tiny Molten Droplets, Dusty Clouds, and Planet Formation). Conel Alexander (Carnegie Institution of Washington) and Jeff Cuzzi (NASA Ames Research Center) summarized the observations. The problem is that keeping the sodium and potassium from escaping requires a substantial enrichment of solids over the pervasive solar nebula gas, and it is not clear how this can be accomplished. Frank Richter (University of Chicago) and colleagues reported on experiments that show that the isotopes of sodium and potassium would have been strongly fractionated if lost by evaporation unless the whole system was partially closed. In other words, the concentrations of these volatile gases were in equilibrium with the molten chondrules. No such fractionations are observed, implying a high dust/gas ratio. However, physical models suggest that creating such a large increase in the dust concentration is unlikely, or at least rare, in the disk. One possibility for enrichment in dust is impact between two objects. In general, the details of such models have not been worked out in sufficient detail to evaluate them quantitatively. An exception to this is modeling by Eric Asphaug (University of California, Santa Cruz) and colleagues. They examined the outcome of collisions between largely molten planetesimals, with droplets ejected into space because of the release of hydrostatic pressure after an impact. Asphaug and coworkers have not evaluated the compositions and range in compositions produced, but the results are interesting.
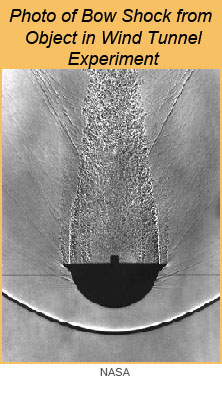
Another interesting mechanism for chondrule formation is the compression of the gas-dust cloud in front of a fast-moving planetary embryo. Such bodies are Moon to Mars sized objects formed rapidly early in Solar System history. These interact gravitationally with each other and with Jupiter and eventually accrete to form the inner planets. Their orbits are not circular, which gives them high relative velocities compared to the nebular gas. As a planetary embryo plows through the gas, it creates a shock in front of it (called a "bow shock"), compressing and heating the dust and gas, possibly leading to the formation of chondrules. Melissa Morris (Arizona State University) and colleagues modeled the bow shock surrounding a Mars-sized object as it plowed through the nascent Solar System (see illustration below). They point out that a recent cosmochemical analysis by Nicolas Dauphas and colleagues (University of Chicago) strongly suggests that Mars formed in 2-4 million years, while the gas was still present. Maybe some chondrules formed when Mars roamed the inner Solar System!
 |
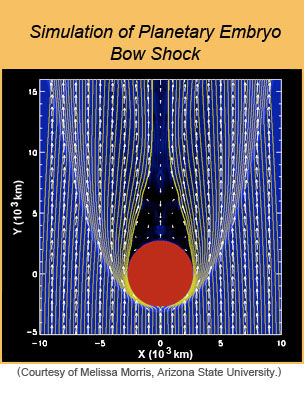 |
| [Left] This photo shows the shock wave in front of a blunt object (moving from top to bottom) in a wind tunnel during an experiment at NASA Ames Research Center. This may resemble the shock wave preceding a planetesimal moving through the solar nebula. [Right] Computer simulation by Melissa Morris and colleagues of a Mars-size embryo moving through the dusty solar nebula. Chondrules very close to the embryo, within about 85 km, accrete to it, but those outside this region are embedded in a gas that contains levels of sodium and other volatiles at high partial pressures, ensuring that the newly formed chondrules do not lose substantial amounts of it while molten. The chondrules formed in the outer region later accrete to planetesimals, making chondrites. | |
Oxygen Isotopes: Galactic Chemical Evolution, Mixing and Modification in the Solar System
Oxygen is the third most abundant element in the Solar System, exceeded only by hydrogen and helium, which reside mostly in the Sun. Variations in its isotopic composition are, therefore, a big deal. Oxygen has three isotopes with masses of 16, 17, and 18. They formed in stars throughout the history of the Galaxy. 16O is by far the most abundant isotope, making up more than 99% of the oxygen. It formed by hydrogen and helium fusion in stars, whereas 17O and 18O are secondary products in stellar evolution, requiring carbon, nitrogen and 16O to be already present. It turns out that the ratio of the secondary products, 17O and 18O, to 16O grows with time.
Considering that oxygen is such an abundant element, you'd think that its isotopic composition would be uniform. But it is not uniform in Solar System materials. It ranges widely among chondrite components (CAIs, chondrules, fine-grained matrix materials) and among different types of differentiated meteorites. Even Mars and Earth have distinctive mixtures of oxygen isotopes. Where does all this heterogeneity come from? This has been a topic of intense discussion for a long time, and the First Solids workshop had its share of discussion of oxygen isotopes in every session, even when the session topic did not concern oxygen. You cannot get away from oxygen isotopes.
One source of variation in oxygen isotopes could be addition to the interstellar cloud in which the Solar System formed, changing the composition of the dust and gas present. In fact, the Sun and the present-day interstellar medium differ in oxygen isotopic composition, which according to Ed Young (University of California, Los Angeles) is consistent with enrichment of the region in which the Sun formed by one or more type II supernovae. However, Larry Nittler (Carnegie Institution of Washington) suggested that supernova addition to the cloud is not necessarily required. The important point is that oxygen isotopes may record a long history of stellar and Galactic evolution.
Of course, processes in the Solar System also alter oxygen isotopes. The large range in Solar System materials may represent differential mixing of two reservoirs, an idea put forth at the conference by Hisayoshi Yurimoto (Hokkaido University, Japan). The sources may be primary, or as many have suggested, caused by a process in the protosolar molecular cloud or protosolar disk such as so-called "self-shielding," that enriches water ice in the heavier isotopes (17O and 18O). For more details, see PSRD article: New View of Gas and Dust in the Solar Nebula.
Whatever the cause of oxygen isotopic variability, it is a useful tool. One particularly interesting application is to follow the history of planetary materials in the Solar System. For example, Justin Simon (Johnson Space Center, Houston) and colleagues discussed CAIs that clearly formed in a place with one oxygen composition, but then traveled to one or more places with different oxygen isotopic compositions. This points towards a dynamic history of particle migration in the Solar System. See PSRD article: A Traveling CAI.
Traveling Through Space and Time
The Formation of the First Solids in the Solar System workshop highlighted the amazing and profound information tied up in Solar System materials. Cosmochemists can look at little objects made in the Solar System and relate them to the processes that made them in the young Solar System and in the galaxy beyond!
- Meteoritics & Planetary Science volume 47, no. 12: Table of Contents and abstracts for the special issue featuring research presented at the Workshop on Formation of the First Solids in the Solar System.
- W. M. Keck Cosmochemistry Laboratory at the Hawai‘i Institute of Geophysics and Planetology, University of Hawai‘i.
- Workshop on Formation of the First Solids in the Solar System (November 7—9, 2011) Full program and listing of abstracts
|
|
[ About PSRD | Archive | CosmoSparks | Search | Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |
