Citation: Taylor, G. J. (April, 2019) Volatile Elements Test Models for the Origin of the Moon, PSRD, http://www.psrd.hawaii.edu/April19/moon-formation-vse.html.

|
April 24, 2019
Volatile Elements Test Models for the Origin of the Moon
--- Volatile elements that concentrate in metallic iron elucidate the processes that operated during formation and initial differentiation of the Moon.
Written by G. Jeffrey Taylor
Hawai'i Institute of Geophysics and Planetology
Siderophile elements concentrate in metallic iron, providing a record of separation of metallic iron from silicate rock or magma, such as during core formation. Volatile elements provide information about the temperatures experienced by planetary materials, such as during large, energetic impacts. Assuming a giant impact origin for the Moon, Kevin Righter (NASA Johnson Space Center) combined these two informative properties to examine the processes that may have been involved in formation of the Moon. Righter considered the initial concentrations of volatile elements in the planetesimals from which Earth formed, loss and mixing of volatiles in an Earth-encircling disk after a giant impact, and formation of a core inside the Moon. The investigation also examined the geochemical consequences of lunar formation resulting from the high-energy collision of proto-planets that created a huge, vaporized, doughnut-shaped object called a synestia. Righter used the latest measurements (including his own extensive work) on the extent to which each of a set of volatile elements concentrate into metallic iron and how easily they are lost due to heating. Assuming Earth and giant impactor began with the same concentrations of 14 volatile siderophile elements (VSEs), Righter's calculations show that the best fit for VSE concentrations in the Moon are for cases that involve a combination of mixing in the proto-lunar disk around Earth, exchange of elements between gas and dust in the disk, and core formation. The agreement is satisfying, but Righter points out that much more work needs to be done to truly unravel the candidate processes leading to formation of the Moon.
Reference:
- Righter, K. (2019) Volatile Element Depletion of the Moon–The Roles of Precursors, Post-impact Disk Dynamics, and Core Formation, Science Advances, v. 5(1), doi: 10.1126/sciadv.aau7658. [ article ]
- PSRDpresents: Volatile Elements Test Models for the Origin of the Moon --Short Slide Summary (with accompanying notes).
Some Chemical Observations about Earth and the Moon
Lunar scientists have known since the mid-1970s that the relative abundances of the three isotopes of oxygen are similar in Earth and the Moon. In fact, as analytical techniques to measure oxygen isotopic composition improved over the decades, the measured isotopic differences have become progressively smaller. In short, the oxygen isotopic abundances are the same (within a few parts per million) in Earth and the Moon. This is important because the oxygen isotopic compositions of most other extraterrestrial materials such as Mars meteorites, chondritic meteorites, and differentiated meteorites differ significantly in oxygen isotopic composition from Earth and the Moon. The diagram below shows one example of oxygen isotopic differences among extraterrestrial materials. This informative diagram plots Δ 17O versus δ 18O. The δ 18O is simply the ratio of 18O to 16O, but normalized to the Earth value as represented by standard mean ocean water (SMOW). The Δ 17O (called "big delta O-17" by cosmochemists) is simply a measure of the vertical displacement of any point from the terrestrial fractionation line on a plot of δ 17O versus δ 18O. Anything exactly like the Earth falls on the dashed line. Note that all the points for the Moon fall on that line, indicating that the Moon has the same oxygen isotopic composition as does the Earth. Mars is distinctly different, having much higher Δ 17O. (For more information about oxygen isotopic variation and how to make sense of it, see PSRD article: Oxygen Isotopes Give Clues to the Formation of Planets, Moons, and Asteroids and for hints about how such data are plotted, see the PSRD primer: Oxygen Isotope Plot.
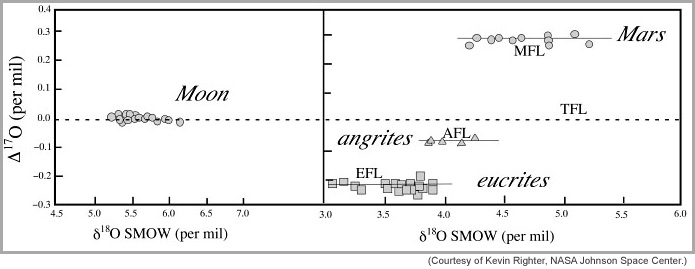 |
| Precise oxygen isotope data for Earth (dashed line), Moon, Mars, asteroid 4 Vesta (eucrite meteorites), and the igneous angrite meteorites. The data for each body spread out along the x-axis because of chemical processing, but all lie close to a line with distinctive big delta O-17. This shows that oxygen isotopic compositions vary throughout our Solar System. TFL, MFL, AFL, and EFL stand for terrestrial, Mars, angrite, and eucrites fractionation lines. |
Oxygen is not the only element with isotopic composition the same in Earth and the Moon. Studies have shown that the isotopic compositions of several refractory elements (e.g., magnesium, chromium, titanium, iron, silicon, tungsten, and ytterbium) are also essentially identical in Earth and Moon. (This conclusion is a bit tricky for tungsten, as explained in PSRD article: Tungsten Isotopes, Formation of the Moon, and Lopsided Addition to Earth and Moon.) And, like oxygen, the isotopic compositions of refractory elements vary throughout our Solar System. The essentially identical isotopic compositions of Earth and Moon coupled with the distinct variation among everything else–Mars, differentiated asteroids (as sampled by meteorites), chondrites and their components–implies either that Earth and Moon formed from a cluster of planetesimals containing refractory elements and oxygen that had the same isotopic compositions as Earth and Moon, or that the Moon-forming giant impact provided conditions that led to the final products having strikingly similar isotopic compositions. More on this below.
A Background on Element Behavior
Elements make up everything around us, such as the dirt decorating our fingernails after gardening and even our fingernails, the rocks in mountain peaks, red-hot lava, cars, the gasoline that fuels old-fashioned vehicles, the lithium in the rechargeable batteries that power modern vehicles, planets, stars, galaxies, interstellar dust, and the gases in the air we breathe (oxygen, nitrogen, argon, and the ever-increasing carbon dioxide). Those elements behave differently from each other, some readily forming compounds with oxygen, others combining with sulfur or metallic iron. Elements behave differently in different environments. For example, the core of Earth is composed mostly of metallic iron, the mantle has iron oxidized to the 2+ valence state, and the crust contains both 2+ and 3+.
Different properties under different chemical environments make elements highly informative about how rocks and even whole planets formed, though it is usually tricky to extract that information. Cosmochemists divide the elements into two main categories, refractory and volatile. The refractory elements are the first to condense from a gas with the composition of the bulk Solar System and are abundant in calcium-aluminum-rich inclusions (CAIs) in carbonaceous chondrites. Calcium and aluminum are refractory elements, as are the rare earth elements, zirconium, uranium, thorium, iridium, platinum, and others. (The elements with identical isotopic compositions in Earth and the Moon are all refractory.)
All the other elements display some volatility, but vary significantly in how volatile they are. The more volatile they are, the more easily they are lost from molten planetesimals and planets. Basically, if you took a bucket of crushed up terrestrial basalt (the main lava type making up oceanic islands) and heated it, highly volatile elements such as hydrogen (in the form of H2O), carbon, and sulfur would begin to stream out (often bound to other elements), followed by somewhat less volatile elements such as zinc, arsenic, bismuth, and thallium. Additional heating to higher temperatures causes loss of alkali elements such as potassium and sodium. This type of behavior can be tracked by measuring the abundance of elements with different volatility in rocks. We have known for a long time that volatile elements are depleted in the Moon compared to their abundances in Earth. For example, in the Moon, the alkali elements sodium, potassium, rubidium, and cesium have 20% of the concentrations they have in Earth.
Besides volatility, elements also have affinities for different types of rocks and minerals. This was developed during the 1930s by Victor Goldschmidt (born in Switzerland, educated mostly in Norway). Goldschmidt divided elements into four groups based on their usual affinity: lithophile, which means rock-loving; siderophile, iron-loving (meaning metallic iron); chalcophile, ore-loving, but in practice elements that have an affinity for sulfur compounds; and atmophile, which are elements that tend to be liquid or gaseous at conditions at Earth's surface. Volatile and refractory behavior can be combined with the Goldschmidt classification. For example, some siderophile elements are refractory and others are volatile to varying degrees. Kevin Righter uses volatile siderophile elements as tools to track processes during lunar formation and formation of the metallic core.
The table below shows element behavior according to Goldschmidt. In each classification, elements can be refractory or exhibit a range in volatility.
| CLASSIFICATION | AFFINITY | WHERE ELEMENTS ARE FOUND IN PLANETS |
| Siderophile | metallic iron | core |
| Lithophile | oxides / silicates | mantle and crust |
| Chalcophile | sulfur | mostly in core |
An interesting side note: These important behavioral properties of elements are captured in one of humanity's greatest inventions, the Periodic Table of the Elements. That great intellectual feat is celebrating its 150 birthday this year. See PSRD CosmoSparks report: 2019: International Year of the Periodic Table.
Building the Moon
The lovely shape-changing globe we see in the night sky formed as the result of a dramatic, energetic event of a giant impact followed by crystallization of a huge, globe-encircling magma from which metallic iron dribbled downwards to form its core. In his paper, Kevin Righter tries to see through all that to understand the details of Moon formation, using what we know about the composition of the Moon from detailed studies of lunar samples.
Since the Conference on the Origin of the Moon in 1984, almost all planetary scientists have concluded that the dramatic event that made the Moon was a giant impact. (Read my reminisces about the conference.) The giant impact theory has evolved over time with numerous numerical experiments involving a range of impactor and proto-Earth sizes, a range in the number of lunar-forming impactors, impactor speeds, angle of impact with the proto-Earth, and how fast Earth and impactor(s) were spinning (hence the total amount of angular momentum).
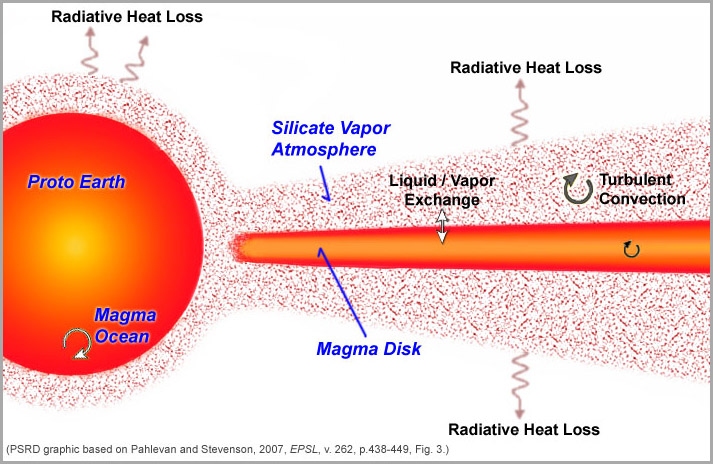
Investigations of the physics of giant impacts lead to two distinctive scenarios for forming the Moon after the impact. One set of models leads to formation of a complicated, hot disk surrounding the proto-Earth, which is composed of vaporized rock and molten droplets that formed during the impact. A newer model involves energetic impacts between proto-planets that produce not a disk, but a huge spinning and glowing mass of molten and vaporized rock. We will examine the traditional, disk model first. The inner region of the proto-lunar disk was investigated by Kaveh Pahlavan and David Stevenson (Caltech). They emphasized the need to somehow end up with the same oxygen isotopic composition in both Earth and the Moon, assuming that the impactor and proto-Earth had different oxygen isotopic compositions. They suggest that after the giant impact and before the Moon formed, Earth exchanged materials with the disk of magma and gas surrounding it, ironing out differences in their isotopic compositions. The process would take 100 to 1000 years, the time they estimate the disk would last before coalescing into the Moon. Their model of the disk is instructive as it highlights the processes operating, including disk cooling, exchange of disk material with the proto-Earth, and turbulent mixing in the melt-vapor cloud. For more details, see PSRD article: Compositional Balancing before Moon Formation.
|
| Proto-Earth and the proto-lunar disk immediately after the giant impact, as depicted by Pahlevan and Stevenson, based on a concept Dave Stevenson published in 1987. Rapid loss of heat into space causes convection in the Earth, the magma disk, and the silicate atmosphere, all of which facilitate exchange of material to even out differences in isotopic and chemical compositions. The silicate atmosphere would act as the exchange medium between Earth and the proto-lunar disk. The Moon-forming region is on the extreme right and beyond (see below). |
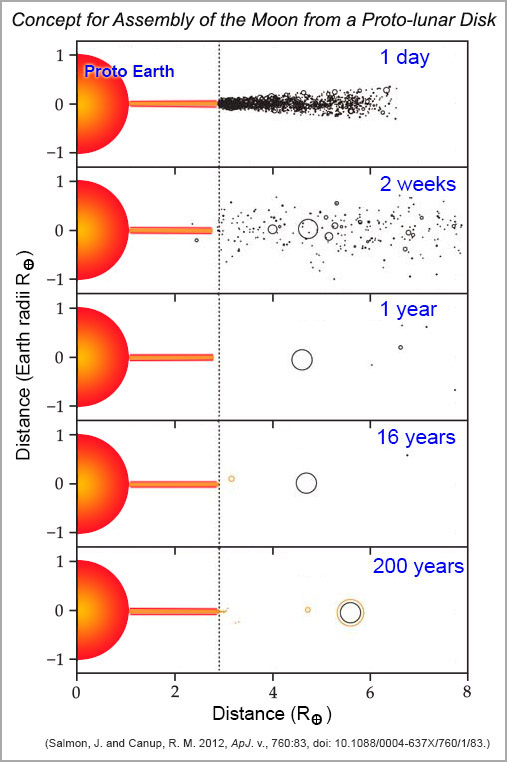
Recent studies of formation of the proto-lunar disk by Julien Salmon and Robin Canup (Southwest Research Institute, Boulder, Colorado) describe the disk and processes in it, including accretion of the Moon. In their model, the Moon forms following a giant impact, as ejecta from the mantles of the proto-Earth and impactor form a proto-lunar disk extending from nearly Earth's surface to beyond 5 RE (Earth radii), more or less the same initial conditions as described by Pahlevan and Stevenson. The Roche limit at 2.9 RE forms an important dynamical boundary. Beyond the Roche limit, heating is limited and cooling is rapid. Calculations reveal that the disk should cool by radiation and vapor should condense on timescales of weeks to months. Gravitational instabilities form moonlets of mass ~0.001 ML (lunar masses) each, which should coalesce into a proto-Moon of mass ~0.4 L. Inside the Roche limit, gravitational instabilities continuously form moonlets that are tidally sheared apart. This shearing produces heat, preventing cooling for ~100 years. This part of the disk spreads beyond the Roche limit and moonlets are spawned just outside the Roche limit, then tidally migrate outward to join the proto-Moon, adding relatively hot, volatile-depleted material to the Moon's outer layers. A paper by Robin Canup, Julien Salmon, Channon Visscher (Southwest Research Institute, Boulder) and Bruce Fegley (Washington University in St. Louis) successfully models the depletions of sodium and potassium in lunar samples.
|
| Moon formation according to Salmon and Canup. Within the shockingly short time of one day, a disk of vaporized and molten rock (orange) stretches out from the molten proto-Earth. Moonlets (black) form in the portion of the disk beyond the Roche limit. The disk within the Roche limit is similar to the one envisioned by Pahlevan and Stevenson (previous diagram), and may only have been a few kilometers thick. Gravity forces drive the moonlets outwards. Interactions are so frequent that about 40% of a Moon mass accretes into one object within a year. The accretion process changes the gravitational configuration and leads to a compression of the disk (orange) inwards (closer to Earth than the Roche limit), preventing formation of additional moonlets near the Roche limit and hence formation of additional moonlets. This moonlet-poor period lasts a bit over a decade, then starts up again. During the next couple of centuries additional material from the hot, volatile-depleted inner disk enters the Moon-forming region and joins the Moon. |
The important point for Kevin Righter's geochemical modeling of volatile siderophile elements is that this complex scenario involves (1) mixing of hot, volatile-depleted materials with cooler materials not depleted in volatiles and (2) separation of melt and gas, which would lead to variations in the amount of volatile elements added to the Moon.
Another fascinating way to form the Moon is in a very energetic collision between two large bodies where the final object ends up substantially vaporized and with substantial angular momentum. This newer idea was devised by Simon Lock (Harvard University, now at Caltech) and Sarah Stewart (University of California, Davis), elaborated by them and their co-workers. In this case the proto-Earth and an impactor have a total angular momentum exceeding that of the present Earth-Moon system. (Angular momentum is the total amount of planetary spinning.) This requires a way to remove the excess angular momentum. Fortunately for this idea, a means to dissipate the angular momentum was devised by Matija Ćuk (SETI institute), working with Simon Lock, Sarah Stewart, and Douglas Hamilton (University of Maryland). The process involves gravitational interactions among the Moon, Earth, and the Sun.
In this scenario, the highly energetic collision does not produce a relatively thin, spread-out disk. Instead, it creates a huge spinning and glowing mass of molten and vaporized rock, much larger than the size of the proto-Earth described in the other giant impact scenarios. This hot object can have shapes ranging from ellipsoidal, with equatorial narrow fins, to giant doughnuts. Lock and Stewart coined a word for these hypothetical structures: synestia, from the prefix syn, which means together, and the Greek goddess Hestia, who represents hearth and home, and by extension architecture. The Earth-Moon synestia would have consisted mostly of vaporized rock in its interior, where the temperature was over 3000 Kelvin. Some large blobs of molten rocks left over from the large impact could have collided to make larger objects, perhaps triggering accretion of the Moon. The upper surface of the synestia would be cooler (about 2300 Kelvin), still intensely hot and glowing. The extent to which volatiles are lost from the region of the synestia where the Moon formed has not yet been investigated in detail. Simon Lock and colleagues show that the synestia model successfully calculates the depletions of sodium, potassium, and other volatile elements observed in lunar samples.
The important point for Kevin Righter's geochemical modeling of volatile siderophile elements is that the synestia scenario likely involved chemical equilibrium between the silicate gas and associated precipitated melt droplets. Chemical equilibrium might also have occurred in the traditional disk model, too.
Forming a Metallic Core in the Moon
Formation of a metallic iron core in the Moon significantly affects the concentrations of siderophile elements that remain in the silicate mantle, which we have measured indirectly through their concentrations in lunar basalts. When the Moon formed it was substantially if not completely molten. Metallic iron, formed by precipitation from the magma ocean, scarfs up the volatile siderophile elements, hence depleting those elements in the silicate magma. How much depletion in the silicate and how much concentration in the metallic liquid has been studied experimentally, including by Kevin Righter. Experiments show that the ratio of the concentration of a siderophile element in metallic iron to that in a co-existing silicate magma (an important number called the partition coefficient) depends on numerous parameters such as oxidation conditions, temperature, pressure, melt composition, and a thermodynamic factor called the activity coefficient. All of these have been determined through numerous laboratory experiments and can be expressed as empirical equations to predict the geochemical behavior of these elements under specified conditions. This allowed Kevin Righter to calculate the effect of core formation on the concentrations of volatile siderophile elements that remain in the silicate magma ocean, hence end up in the lunar mantle.
Mixing and Matching
Analyses of lunar samples have pinned down the abundances of volatile siderophile elements in the lunar mantle (a lot of other elements, too). These are the concentrations that Kevin Righter used as ground truth, and shown as blue dots in the diagram below. He then tested three different Moon compositions, varying the amounts of volatile elements in the proto-Earth and impactor. Like Righter's paper, we will focus on the one that works best. This one begins with concentrations of volatile elements like those in the initial terrestrial upper mantle. This has been established reasonably well through decades of analyses of Earth basalts and mantle rocks brought to the surface in basaltic magmas. (Earth's initial mantle composition is named the Primitive Upper Mantle (PUM) composition.)
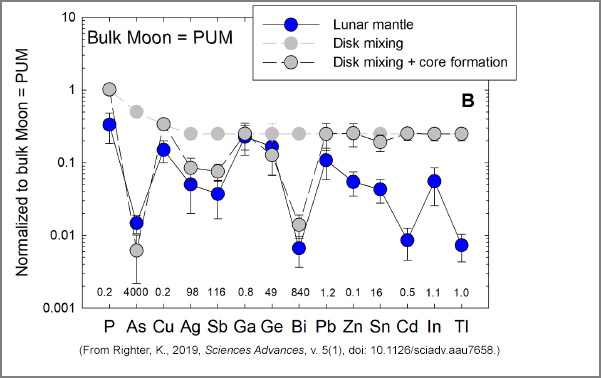
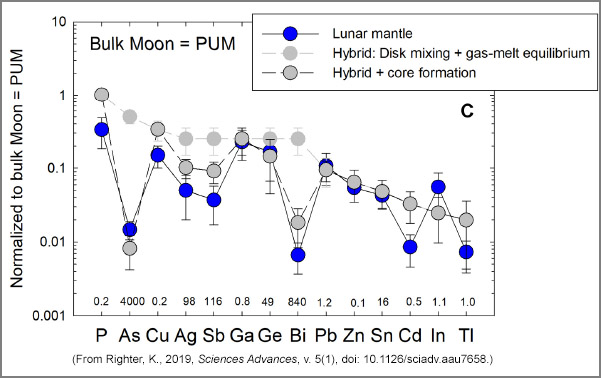
The graphs, below, show how the concentrations of volatile siderophile elements vary with different processes that could have been at work during formation of the Moon and during core formation. The elements are listed on the x-axis in order of increasing volatility (P is the least volatile, Tl is the most volatile). The starting composition is 1 for each element (1 on the y-axis), meaning that the initial concentration is the same as in the primitive upper mantle (PUM) of Earth. The concentrations in the lunar mantle are shown in blue, which we want to match in the calculations. Note that all elements are depleted compared to the PUM composition, demonstrating loss of volatiles during Moon formation and fractionation during core formation. The small numbers along the bottom of each graph list the metal-silicate partition coefficients; 1 indicates that an element has no preference for metal over silicate, <1 indicates that an element is not particularly fond of metallic iron, and >1 indicates an affinity for metallic iron. Note that some elements have partition coefficients much greater than one.

|
ALight gray circles show the effect of gas-melt equilibrium. Arsenic (As) differs significantly from the bulk Moon composition. Dark gray, outlined circles show the combined effects of gas-melt equilibrium and core formation. The large discrepancy in the abundance of As is fixed, but significant differences between calculated and measured lunar mantle compositions are apparent for silver (Ag), Antimony (Sb), bismuth (Bi), and indium (In). |
|
BIn this case, Kevin Righter uses disk mixing (light gray circles) in which the hot inner disk (which experienced volatile loss) mixed with the cooler outer disk (retained its volatiles). Few elements match the lunar mantle composition. With core formation (dark gray, outlined circles) a few elements (As, Ag, Sb, Bi) match reasonably well, but the most volatile elements do not come close to matching the lunar composition. |
|
CThis shows a more complicated model. The light gray circles show the composition produced by a hybrid process combining disk mixing with gas-melt equilibrium. Large discrepancies occur for As and Bi. When this hybrid calculation is combined with core formation (dark gray, outlined circles), the fits, while not perfect, are good for all 14 elements. |
Implications
Kevin Righter's calculations of the history of volatile siderophile elements (and some others) highlight the geochemical events that affected this interesting set of elements. It seems clear that in a proto-lunar disk or synestia both gas-melt equilibrium and the mixing of materials with higher and lower volatile concentrations are required. Core formation also leaves its own record. Segregation of gas and melt in a proto-lunar disk has been proposed to occur by several plausible mechanisms, such as tidal interactions, viscosity differences, and turbulence. More research is needed to explore the mechanisms for gas-melt separation during lunar formation in a synestia.
The important lesson in Righter's work is that geo- and cosmochemical principles can be used to develop tests of the complicated process of lunar formation. The large group of volatile elements used in Righter's analysis are particularly useful in constraining these diverse processes. If it can be shown that the Moon formed in a synestia, the lunar composition can be used as a recorder of the processes operating during planet formation in a synestia.
- PSRDpresents: Volatile Elements Test Models for the Origin of the Moon -- Short Slide Summary (with accompanying notes).
- Canup, R. M., Visscher, C., Salmon, J., and Fegley, Jr., B. (2015) Lunar Volatile Depletion due to Incomplete Accretion Within an Impact-generated Disk, Nature Geoscience, v. 8, p. 918-921, doi: 10.1038/ngeo2574. [abstract]
- Lock, S. J., Stewart, S. T., Petaev, M. I., Leinhardt, Z., Mace, M. T., Jacobsen S. B., and Ćuk, M. (2018) The Origin of the Moon Within a Terrestrial Synestia, Journal of Geophysical Research: Planets, v. 123, p. 910-951, doi: 10.1002/2017JE005333. [abstract]
- Lock, S. J. and Stewart, S. T. (2017) The Structure of Terrestrial Bodies: Impact Heating, Corotation Limits and Synestias, Journal of Geophysical Research: Planets, v. 122, p. 950-982, doi: 10.1002/2016JE005239. [abstract]
- Righter, K. (2019) Volatile Element Depletion of the Moon–The Roles of Precursors, Post-impact Disk Dynamics, and Core Formation, Science Advances, v. 5(1), doi: 10.1126/sciadv.aau7658. [article]
- Salmon, J. and Canup, R. (2014) Accretion of the Moon Rrom Non-canonical Discs, Philosophical Transactions of the Royal Society A, v. 372: 20130256, doi: 10.1098/rsta.2013.0256. [article]
- Taylor, G. J. (Dec. 1998) Origin of the Earth and Moon, Planetary Science Research Discoveries, www.psrd.hawaii.edu/Dec98/OriginEarthMoon.html.
|
|
[ About PSRD | Archive | CosmoSparks | Search | Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |


