Citation: Taylor, G. J. (December, 2018) The Complicated Origin of Earth's Water, PSRD, http://www.psrd.hawaii.edu/Dec18/origin-earth-water.html.

|
December 20, 2018
The Complicated Origin of Earth's Water
--- Water delivery and redistribution to Earth was a complicated business with hydrogen coming from chondritic and solar nebular sources.
Written by G. Jeffrey Taylor
Hawai'i Institute of Geophysics and Planetology
A major mystery in planetary science is the origin of Earth's water. Using the deuterium/hydrogen ratio (D/H) as an isotopic tracer of the origin of water in our planet, Jun Wu (Arizona State University) and colleagues at ASU model the origin of water to Earth. Through quantitative modeling they show that Earth contains about eight-oceans-worth of water, of which about five-oceans-worth reside in the metallic core (as hydrogen). They also show that a minor but important component came via "ingassing" from a proto-atmosphere surrounding Earth, which formed by accretion of solar nebula gas. It would not take much dissolution of hydrogen from this atmosphere to modify the hydrogen isotopic composition of the originally accreted Earth to account for the overall isotopic composition (given by D/H) of the planet. The model also considers dissolution of hydrogen into the metallic core of Earth and its effect on D/H. The research is a good example of the power of using isotopic compositions as tracers of planetary formation and differentiation processes.
Reference:
- Wu, J., Desch, S. J., Schaefer, L., Elkins-Tanton, L. T., Pahlevan, K., and Buseck, P. R. (2018) Origin of Earth's Water: Chondritic Inheritance Plus Nebular Ingassing and Storage of Hydrogen in the Core, Journal of Geophysical Research: Planets, v. 123, p. 2691-2712, doi: 10.1029/2018JE005698. [abstract]
- PSRDpresents: The Complicated Origin of Earth's Water -- Short Slide Summary (with accompanying notes).
Where Did Earth's Essential Water Come From?
Water is essential to our lives. Not only could we not live without it, but we would not even have evolved without it, nor would our fellow travelers on the tree of life have originated and evolved. And we could not go sailing, swimming, scuba diving, surfing, kayaking, or jet skiing in the ocean, which occupies three-quarters of Earth's surface. Even the continents have lakes and rivers large and small. Water even flows beneath the surface as groundwater, seeping through pore spaces in solid rock. Water resides deep in the crust and throughout the mantle. Earth has a big, complicated water cycle that involves not only water evaporation and then condensation into rain, but also dragging water down deep into the mantle in subducting oceanic plates, eventually leading to its reemergence to the surface in volcanic eruptions.
In other words, water is important, and Earth has oceans of it. But where did it come from? This is a long-standing question in planetary science, and one of the most interesting. A popular idea is that most water was delivered by planetesimals similar to carbonaceous chondrite meteorites. The idea stems from the similarity in hydrogen isotopic composition in Earth and carbonaceous chondrites, as shown by Conel Alexander (Carnegie Institution of Washington) and colleagues (see PSRD report: Water, Carbonaceous Chondrites, and Earth). A crucial piece of information in this work and the detail that connects Earth's water to carbonaceous chondrites, is the deuterium/hydrogen ratio (D/H). Deuterium is an isotope of hydrogen that's also called heavy hydrogen. Its nucleus contains a proton and a neutron; hydrogen's nucleus contains only a proton. As a reference, the measured D/H in Earth's standard mean ocean water (SMOW) is 156 x 10-6 (0.000156). Alexander and associates focused on D/H in the water that is bound inside hydrous minerals, which averages 140 x 10-6. This is a bit below the D/H in mean ocean water, leading Alexander and colleagues to conclude that there must have been components of lower, solar D/H materials mixed into Earth, which they suggest are volatile-rich CI-like (with D/H of 64–98 x 10-6) and other materials with isotopically solar compositions.
Hints of a component from the solar nebula (with D/H of 21 x 10-6) comes from study of the hydrogen isotopic composition of water in basalts from Baffin Island, Canada, as shown by Lydia Hallis (now at the University of Glasgow, Scotland) (see PSRD article: Primeval Water in the Earth). Baffin Island basalts, like a few from other places, have the important characteristic of having high 3He/4He, another indicator of a source in the solar nebula. Lydia Hallis and colleagues found that the D/H in the basalts was less than 122 x 10-6, hinting at the presence of a primitive component with even lower D/H. The basalts derive from the deep mantle and their lead (Pb) isotopic compositions indicate formation of their deep mantle sources >4.45 billion years ago, suggesting an ancient, primordial origin for their source region in the mantle—a source that appears to have contained primitive helium and water.
Adding and Modifying Earth's Water in Six Stages
Jun Wu and colleagues at Arizona State University have examined the issue broadly, considering planetary accretion, magma oceans, core formation, mantle convection, and interaction of a proto-atmosphere with the surrounding solar nebula. Their comprehensive hypothesis uses the isotopic composition of hydrogen as a tracer of the sources of water and its processing during planetary formation and subsequent differentiation. Here is an overview of their model in six stages.
 |
|
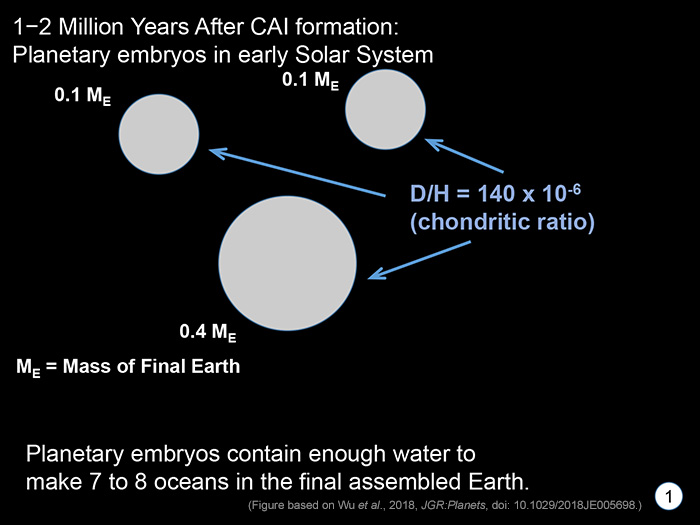
STAGE 1 The first step occurred long before there was an Earth, about 1–2 million years after formation of CAIs, the first solids to form in the Solar System. During this time large objects (called planetary embryos) assemble with masses up to a few tenths of Earth's final mass. The idea of such rapid growth is relatively new and is driven by two ideas. One is called streaming instability, which leads to growth of meter-sized aggregates to 100-km planetesimals in less than a million years. The other is nicknamed pebble growth, which starts with meter-sized (or smaller) objects and ends up with large planetary embryos in less than a million years. This short time scale is supported by age dates on assorted types of differentiated meteorites and by the time of core formation in Mars (based on tungsten isotopes in Martian meteorites), only 1.8 million years after CAIs (as shown by Nicholas Dauphas and A. Pourmand in 2011). So, Wu and coworkers assume that it takes only 1-2 million years to form a bunch of planetary embryos. They also assume that the D/H in them is the same as observed in water-bearing minerals in carbonaceous chondrites, about 140 x 10-6 (it could range from 130–170 x 10-6). |
 |
|
STAGE 2 Then the planetesimals melted. Growing planetesimals began heating as they were forming. The heat came from the decay of 26Al, one of the isotopes of aluminum, an abundant element. When radioactive elements decay, they release a particle such as an electron and some energy, which heats up its surroundings. 26Al has a half-life of only 730,000 years, which means, of course, that this original 26Al is gone now. (We know it existed from excesses in the amount of 26Mg in aluminum-bearing minerals in chondrites and other ancient materials.) The short half-life makes 26Al a potent heat source, so strong that it can melt a planetesimal as small as about 10 kilometers in radius. Larger planetesimals melt readily, allowing metallic iron in them to drain to their cores. As the metallic dribbling begins, some hydrogen readily dissolves in metal droplets. Wu and coworkers calculate, based on published hydrogen solubility in metallic iron, that the cores of the planetary embryos would contain about 0.025 to 0.055 wt% hydrogen, which corresponds to three- to six-oceans-worth in the metallic core of the final assembled Earth. In addition, H and D do not enter the metallic core in equal proportions. Based on experiments on H and D dissolution and the chemical principles of isotopic fractionation, Wu and colleagues conclude that the cores of the embryos would have D/H of 125–135 x 10-6. This lower ratio would increase the D/H in the mantles of the embryos to 150–160 x 10-6. |
 |
|
STAGE 3 A planetary embryo surrounded by an ocean of magma could attract an atmosphere from the solar nebula, forming a stable (though evolving) proto-atmosphere. Jun Wu and colleagues suggest that this atmosphere could interact with the magma ocean to incorporate water with a lower D/H than that produced during stages 1 and 2. This process has been dubbed "ingassing." Most investigators have concluded that this mechanism is not particularly effective. Exceptions are papers by Marc Hirschmann (University of Minnesota) and colleagues in 2012 and Zack Sharp (University of New Mexico) in 2017. However, Jun Wu and his coworkers show that this process could be effective enough to lower the average D/H in the magma ocean surrounding a planetary embryo to about 109 x 10-6. It would provide about 0.14 oceans of hydrogen depending on the magnitude of partitioning of H and D between iron melt and silicate mantle. The exact amount of water in the magma ocean is uncertain, hence the D/H ratio is also uncertain. Nevertheless, it is likely that D/H in the magma ocean would have been lower than originally present in a planetary embryo. The ingassing process would have operated early, within 3 million years after CAI formation. This is the time the solar nebula lasted, as shown by astronomical observations of disks around young stars and by the time of Mars formation (1.8 million years after CAIs). |
 |
|
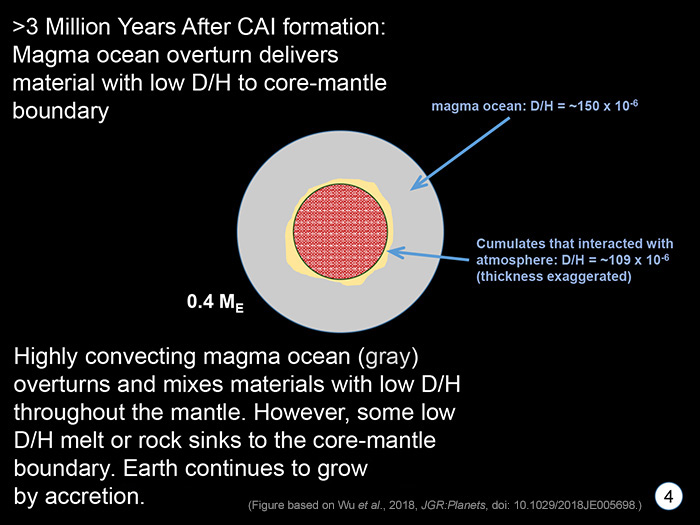
STAGE 4 A magma ocean does not simply solidify into a rock-solid mantle. The crystallization process produces an unstable pile of rock, with some admixed melt, with denser minerals on top and less dense ones below. The consequences of this density stratification is that the pile overturns. It turns over in a decided messy way. (See PSRD article: A Primoridal and Complicated Ocean of Magma on Mars.) The overturn would mix patches of magma ocean with differing D/H due to incomplete mixing of the low D/H hydrogen in the proto-atmosphere into the magma ocean and the complicated dynamics of the overturn. Some crystallized piles of crystals (called cumulates) would have acquired the magma ocean's D/H (estimated by Wu and colleagues to have D/H of 109 x 10-6) and could have settled to the core-mantle boundary. These might later rise in plumes to form lavas with high 3He/4He and low D/H. |
 |
|
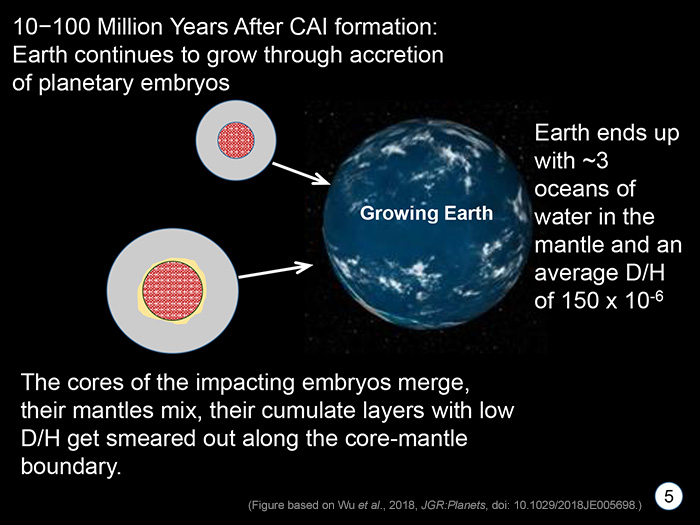
STAGE 5 During the next 10 to 100 million years Earth continued to grow through accretion. The accretion process is not gentle as the objects smash into each other, mixing their mantles and cores, possibly smearing out the low D/H cumulates along the core-mantle boundaries. |
 |
|
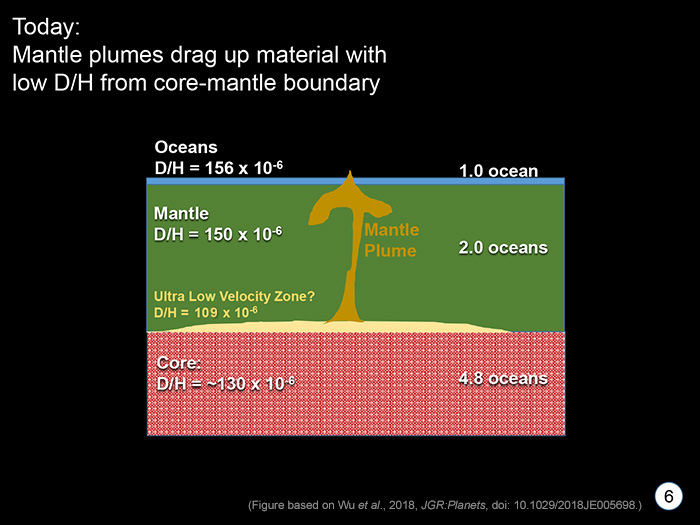
STAGE 6 (TODAY) From about 4.4 billion years ago to now, Earth has done its Earth thing, forming continents, starting plate tectonics, moving water around inside and on top of it. Some surviving regions with low D/H deep in the mantle were incorporated into ascending plumes of buoyant rock that rose up to eventually melt and produce basalts like we see in Baffin Island, Canada. Most magmas that erupt onto the surface of our planet reflect more average mantle, having D/H around 150 x 10-6. Wu and coauthors estimate that Earth contains almost eight-oceans-worth of water in it, about 60% of it in the metallic core (dissolved as hydrogen). |
Earth's water holds a rich record of planetary formation, melting, differentiating into core and mantle, and interaction with the primitive solar nebula. The evidence is tied up in the isotopic composition of hydrogen, coupled with other volatile species such as neon, as shown by Williams and Mukhopadhyay (University of California, Davis), and helium. Similar stories of planetary accretion and differentiation are told by other isotopic systems, such as tungsten isotopes (see PSRD report: Hafnium-Tungsten Isotopes). This work by Jun Wu and others relies on the availability of samples from many objects in the Solar System and places on Earth, and on the continued advancements in technology and instrumentation for determining isotopic compositions.
- PSRDpresents: The Complicated Origin of Earth's Water --Short Slide Summary (with accompanying notes).
- Alexander, C. M. O'D., Bowden, R., Fogel, M. L., Howard, K. T., Herd, C. D. K., and Nittler, L. R. (2012) The Provenances of Asteroids, and Their Contributions to the Volatile Inventories of the Terrestrial Planets, Science, v. 337(6095), p. 721-723, doi: 10.1126/science.1223474. [article].
- Dauphas, N. and Pourmand, A. (2011) Hf-W-Th Evidence for Rapid Growth of Mars and its Status as a Planetary Embryo, Nature, v.473, p. 489-492, doi: 10.1038/nature10077. [abstract]
- Hallis, L. J. (2017) D/H Ratios of the Inner Solar System, Philosophical Transactions of the Royal Society A, v. 375(2094), 20150390, doi: 10.1098/rsta.2015.0390. [article]
- Hallis, L. J., Huss, G. R., Nagashima, K., Taylor, G. J., Halldörsson, S. A., Hilton, D. R., Mottl, M. J. and Meech, K. J. (2015) Evidence for Primordial Water in Earth's Deep Mantle, Science, v. 350(6262), p. 795-797, doi: 10.1126/science.aac4834. [abstract]
- Hirschmann, M. M., Withers, A. C., Ardia, P., and Foley, N. T. (2012) Solubility of Molecular H in Silicate Melts and Consequences for Volatile Evolution of Terrestrial Planets, Earth and Planetary Science Letters, v. 345-348, p. 38-48, doi: 10.1016/j.epsl.2012.06.031. [abstract]
- Martel, L. (2017) Hafnium-tungsten Isotopes, PSRD CosmoSparks, http://www.psrd.hawaii.edu/CosmoSparks/Oct17/Hf-W-isotopes.html.
- Martel, L. (2012) Water, Carbonaceous Chondrites, and Earth, PSRD CosmoSparks, http://www.psrd.hawaii.edu/CosmoSparks/July12/Earthwater-sources.html.
- Sharp, Z. D. (2017) Nebular Ingassing as a Source of Volatiles to the Terrestrial Planets, Chemical Geology, v. 448, p. 137-150, doi: 10.1016/j.chemgeo.2016.11.018. [abstract]
- Taylor, G. J. (2015) Primeval Water in the Earth, PSRD, http://www.psrd.hawaii.edu/Nov15/Earth-primeval-water.html.
- Taylor, G. J. (2006) A Primordial and Complicated Ocean of Magma on Mars, PSRD, http://www.psrd.hawaii.edu/Mar06/mars_magmaOcean.html.
- Williams, C. D. and Mukhopadhyay, S. (2018) Capture of Nebular Gases During Earth's Accretion is Preserved in Deep-mantle Neon, Nature, doi: 10.1038/s41586-018-0771-1. [abstract]
- Wu, J., Desch, S. J., Schaefer, L., Elkins-Tanton, L. T., Pahlevan, K., and Buseck, P. R. (2018) Origin of Earth's Water: Chondritic Inheritance Plus Nebular Ingassing and Storage of Hydrogen in the Core, Journal of Geophysical Research: Planets, v. 123, p. 2691-2712, doi: 10.1029/2018JE005698. [abstract]
|
|
[ About PSRD | Archive | CosmoSparks | Search | Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |
