Citation: Taylor, G. J. (July, 2012) How Much Water is Inside Mars? Planetary Science Research Discoveries. http://www.psrd.hawaii.edu/July12/water-inside-Mars.html (date accessed).
 |
July 31, 2012
How Much Water is Inside Mars?
--- The interior of Mars appears to be as wet as the interior of Earth.
Written by G. Jeffrey Taylor
Hawai'i Institute of Geophysics and Planetology
Several lines of evidence indicate that the surface of Mars was sculptured by flowing water and glaciation, but how much water was needed for this planetary art work is not easy to determine. In particular, how much is in the mantle that underlies the basaltic crust of Mars? How much was inside the planet when it formed? Lacking pieces of the mantle, the best way to address this question is to calculate how much water was in magmas produced by melting portions of the mantle. Francis McCubbin and colleagues at the University of New Mexico and the Carnegie Institution of Washington measured the amount of water (actually the hydroxyl molecule, OH) in the mineral apatite in two shergottites, martian meteorites formed by partial melting of the interior. They measured several tenths of a percent by weight H2O in apatite in two shergottites. By making reasonable assumptions about crystallization history and the amount of melting required to produce the shergottite magmas, they estimate that the interior contains between 73 and 290 parts per million H2O, which overlaps the lower range of water estimates for the Earth's mantle. This water was likely incorporated into both bodies as they formed by accretion of smaller bodies.
Reference:
- Francis M. McCubbin, Eric H. Hauri, Stephen M. Elardo, Kathleen E. Vander Kaaden, Jianhua Wang, and Charles K. Shearer, Jr. (2012) Hydrous Melting of the Martian Mantle Produced Both Depleted and Enriched Shergottites. Geology, v. 40(8), p. 683-686, doi: 10.1130/G33242.1.
- PSRDpresents: How Much Water is Inside Mars? --Short Slide Summary (with accompanying notes).
Wet Mars
Mars is decorated with numerous channels and river valleys, which show that liquid water gushed onto and flowed across its surface. In addition to the convincing photogeologic evidence for water processes, the upper meter of the surface contains between 2 and 7 wt% water in a broad equatorial zone and up to 70 wt% in polar regions. In fact, some places have pure ice exposed. These megabuckets of water reacted with primary igneous minerals to form an assortment of clay minerals that have been identified by reflectance spectrometers orbiting Mars and found inside martian meteorites. Most of this planet-wide irrigation occurred during the first billion years or so of the planet's history.
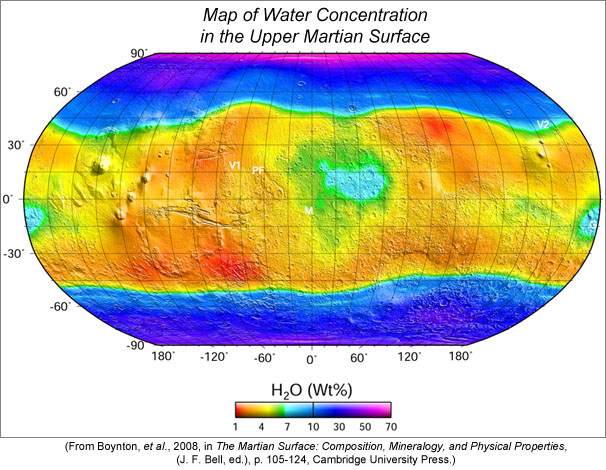 |
| Map of the H2O concentration in the upper few tens of centimeters of the martian surface, as measured from orbit by the Mars Odyssey Gamma Ray Spectrometer (GRS). Equatorial Mars (about 45 degrees north and south) contains between 2 and 7 wt.% H2O, and polar regions contain much more. The GRS actually measured hydrogen (H), but those concentrations have been converted to H2O. In reality, much of the water may be in the form of OH bound in minerals. |
 |
| Map showing places on Mars where remote sensing specialists used visible-infrared data from the Compact Reconnaissance Imaging Spectrometer for Mars (CRISM) instrument on the Mars Reconnaissance Orbiter to investigate small regions in a search for clay minerals. Such minerals are formed when water reacts with anhydrous igneous minerals. The base map is a shaded relief map. Clay minerals were identified in numerous places and the symbols are color-coded by geological setting. The important point is that the observations show that water-bearing clay minerals are widespread on the surface. The open symbols mark places where no clays were found. |
The evidence is clearly overwhelming that the martian surface contains water, and clay minerals probably occur throughout the 50-kilometer thick crust. The water most likely came from the interior, delivered by magmas, but it is not clear how much water remains inside Mars, how much was there in the first place, and how it got there. Water could have been delivered to Mars as it was growing by accretion of planetesimals, or it might have been added after the planet was mostly constructed. McCubbin and his colleagues studied martian meteorites to shed light on these questions.
Probing the Martian Mantle
One would think that the martian mantle will remain deep and mysterious without samples of it. Not so. The interior is hot and in its upper reaches the pressure is low enough to melt. Because rocks melt over a range of temperatures (a few hundred degrees Celsius), the mantle only partially melts. Molten material at first coats the interfaces between crystals, forming films, then small channels, then bigger channels as the buoyant melt rises. The melts (magmas) formed by partial melting contain a surprisingly good record of the composition of the rocks that melted, so if we had samples of those melts we could determine a lot about the mantle. Fortunately, we do have samples: lava flows that erupted onto the surface. Cosmochemists have to contend with a few complications, of course, such as partial crystallization in the cooler crust and reaction of the magmas with the crust. These obscuring processes leave their own types of chemical fingerprints so cosmochemists can disentangle the secondary from the primary compositional features. Or at least mostly disentangle them.
McCubbin and coworkers studied two basaltic lava flows. Both are in the shergottite class of martian meteorites. Cosmochemists have identified two flavors of shergottites: enriched and depleted. A few samples, of course, are in between these extremes. The groups vary in their chemical and isotopic compositions. The enriched group contains higher concentrations of light (low atomic number) rare earth elements than does the depleted group. The simple diagnostic is the ratio of lanthanum (La) to ytterbium (Yb); enriched shergottites have La/Yb of 1.5 (similar to that in chondrites) and the depleted ones have La/Yb of 0.1. Along with these are variations in oxidation state and initial ratios of strontium and neodymium isotopes. The groups indicate distinctive mantle source regions (the places that partially melted), although it is possible that the enriched group magmas reacted with crustal rocks during their journey to the surface. See the PSRD article: The Multifarious Martian Mantle for more details about the enriched and depleted groups.
A few shergottites have been analyzed for H2O. These indicate relatively low water contents (only 50-150 parts per million), but these are not reliable because H2O is not soluble in magma at the low pressure as experienced during eruption onto the martian surface. (It is surprisingly soluble in magma at high pressure, containing several percent at pressures experienced greater than a few kilometers beneath the surface.) The better way to determine water contents of magmas is to measure it in a mineral that contains it bound in its crystal structure. McCubbin and colleagues focused on the mineral apatite.
An Apatite for Water
Apatite is a phosphate mineral with the ideal formula of Ca5(PO4) 3 (F,Cl,OH), in which hydrogen is bound as the OH- ion. This mineral is actually even more complicated than it appears, with rare earth elements prone to substitute in the site occupied by calcium (Ca), and sulfur sneaks into the site occupied by fluorine (F), chlorine (Cl), and OH. Its only real flaw is that it crystallizes late when lavas solidify, allowing time for water to leak out of the lava. Nevertheless, apatite OH content allows us to estimate the minimum water content of the pre-eruption magma.
McCubbin and colleagues determined the OH content of apatite by analyzing H in a secondary ion mass spectrometer (SIMS). They also measured F, Cl, and S by SIMS. They determined the other elements and repeated F, Cl, and S by electron microprobe. The SIMS project involved creating a set of natural apatite standards with independently determined OH concentrations. Francis McCubbin generously shares these important materials with other cosmochemists.
 |
 |
| [LEFT] Picture of Shergotty meteorite on display at the Natural History Museum in Vienna, Austria. [RIGHT] Picture of QUE 94201 shergottite collected by the U. S. Antarctic Search for Meteorites program (ANSMET) in Queen Alexandra Range, Antarctica. | |
The team measured an enriched shergottite (Shergotty, the group's namesake, observed to fall in India in 1865, Data link from the Meteoritical Bulletin) and a depleted shergottite (QUE 94201, found in Antactica, Data link from the Meteoritical Bulletin) to see if their water contents are different like so many other geochemical parameters are. They found that the H2O contents of apatite in Shergotty (enriched) ranges from 0.46 to 0.86 wt% H2O, and in QUE 94201 (depleted) it ranges from 0.22 to 0.64 wt%. (Why, an alert reader might ask, does McCubbin report OH as H2O? Well, that's the way it is done traditionally. When phosphorus (P) is measured in the electron microprobe, it is reported as PO4, but we actually measure just P. When chemical compositions are converted to a mineral's formula, everything is put in atomic fractions, with H calculated as OH.)
Water Content in Shergottite Magmas and Mantle Sources
The water content of the lava in which each sample formed can be calculated from a distribution coefficient determined experimentally. These are wildly important geochemical parameters that tell us how an element or compound, in this case H2O, goes into a crystal (apatite) as a function of its concentration in the melt. If we know this value and we know how much is in the mineral and the distribution coefficient does not vary with concentration, we can determine the amount of H2O in the melt (lava). Too bad we do not know this value for apatite particularly well. Experiments indicate that the ratio of water in apatite to water in the magma it is crystallizing from is somewhere between 0.1 and 0.3. To be conservative and estimate the minimum lava water content, McCubbin and colleagues use the larger value. This results in lava concentrations at the time of apatite crystallization ranging from 1.5 to 2.8 wt% in the Shergotty lava, and from 0.7 to 2.1 wt% in the QUE 94201 magma.
But when did apatite crystallize? This is important because H2O does not go into most minerals and so concentrates in the remaining lava as a flow crystallizes. If apatite crystallized when only 10% of the lava was still molten (90% crystallized), its abundance in the melt would have increased by a factor of 10 (100% / 10% = 10). At 50% crystallization it would have concentrated by only a factor of 2. Based on yet another set of experiments, McCubbin and colleagues use experimental data that indicate that phosphate minerals (there are others besides apatite and they do not contain water) form after 75 to 90% of the lava has crystallized. Using 90% to minimize the estimated water concentration, McCubbin estimates that the Shergotty lava contained, before it began to crystallize, 0.15 to 0.29 wt% H2O, and the QUE 94201 lava contained 0.07 to 0.21 wt%. These are reliable minimum values for the magmas before they erupted not only because the calculations were done to estimate the minimum values, but also because it ignores loss of water during eruption. In fact, it is likely that at least 90% of the water was lost, so the actual initial magma concentrations could be more than about 1 wt% and as much as a few percent.
These calculations involve a lot of assumptions and estimates. Undaunted, McCubbin used them to estimate how much H2O is in the mantle sources that partially melted to produce the Shergotty and QUE magmas. The central uncertainty in this estimate is how much partial melting occurred before the magma segregated and began its trip to the surface. The concentration of water inferred to be in the mantle is proportional to the percentage of partial melting, as shown in the diagram below. Assuming 10% partial melting, McCubbin estimates that the mantle that produced the depleted magma contained between 73 and 210 parts per million H2O and the enriched one contained between 160 and 290 parts per million. For comparison, the terrestrial mantle that produces mid-ocean ridge basalts contains between 100 and 350 parts per million (0.01 to 0.035 wt%).
 |
| Plot showing the estimated water content in the martian mantle for enriched (Shergotty) and depleted (QUE 94201) magmas as a function of the amount of partial melting. Shown for comparison are values estimated for the magma in which the martian meteorite Chassigny formed (data from a previous McCubbin paper), the Earth (blue region, which actually extends down to 100 ppm), and lunar basalts. The purple area at the bottom represents the mantle estimated by Justin Filiberto (Southern Illinois University) and Alan Treiman (Lunar and Planetary Institute, Houston) from whole-rock H2O analyses. The significant overlap (orange region) in inferred mantle H2O contents for enriched and depleted shergottites indicates that water was present in the mantle and likely was present before the source regions formed by primary differentiation. McCubbin and colleagues suggest that Mars formed with its full complement of water and that there is no need to add significant amounts of extra water later. |
When Mars Got its Water
The significant overlap in inferred mantle H2O contents for enriched and depleted shergottites (see diagrams above and below) indicates that water was present in the mantle and likely was present before the source regions formed by primary differentiation. McCubbin and colleagues suggest that Mars formed with essentially its full complement of water and that there is no need to add significant amounts of extra water later. The distinctive chemical signatures of enriched and depleted shergottites almost certainly formed when the planet differentiated from its initially substantially molten state. Isotopic studies show that this big event took place 4.5 billion years ago and that the mantle regions that eventually gave rise to the shergottites did not mix or melt until shergottite formation 180 to 500 million years ago. (See PSRD article: The Multifarious Martian Mantle.) Thus, argue McCubbin and his colleagues, the water was probable present when the source regions formed, hence Mars formed with its water already present. There is no need to add it after primary differentiation.
 |
| Initial neodymium-143 to -144 ratio (normalized to the initial ratio in chondrites and reported as parts per ten thousand, a parameter nicknamed epsilon Nd, εNd) plotted against mantle water content for the two shergottites measured by McCubbin and colleagues (Shergotty and QUE 94201) and other data reported previously by others. Los Angeles and Zagami are enriched shergottites. Chondritic uniform reservoir (CHUR, εNd=0 by definition) is also shown. McCubbin and coworkers argue that the overlap in H2O concentrations combined with the enormous range in εNd indicates that water was already present in Mars when it differentiated 4.5 billion years ago. |
It is fascinating that the mantles of Mars and Earth have similar concentrations of water. Of course, another 250 parts per million H2O is in the terrestrial oceans, but the crust of Mars also has a lot of water (see the GRS H2O map above). I estimate from GRS data that about half the potassium in Mars is in the crust. If that holds for water, then half of the planet's complement of water is in the crust, making the bulk H2O similar in Earth and Mars. Perhaps the two planets formed from a similar mix of planetesimals. Things are not so simple, of course, but it is clear that these new analyses of martian meteorites are providing new windows into the martian mantle and giving fresh insight into how Mars formed.
- PSRDpresents: How Much Water is Inside Mars? --Short Slide Summary (with accompanying notes).
- Boynton, W. V., Taylor, G. J., Karunatillake, S., Reedy, R. C., and Keller, J. M. (2008) Elemental Abundances Determined by Mars Odyssey GRS. In The Martian Surface: Composition, Mineralogy, and Physical Properties (J. F. Bell, ed.), p. 105-124. Cambridge University Press.
- Ehlmann, B. L., Mustard, J. F., Murchie, S. L., Bibring, J.-P., Meunier, A., Fraeman, A. A., and Langevin, Y. (2011) Subsurface Water and Clay Mineral Formation During the Early History of Mars. Nature, v. 479, p. 53-60, doi: 10.1038/nature10582.
- McCubbin,F. M., Hauri, E. H., Elardo, S. M., Vander Kaaden, K. E., Wang, J., and Shearer, Jr., C. K. (2012) Hydrous Melting of the Martian Mantle Produced Both Depleted and Enriched Shergottites. Geology, v. 40(8), p. 683-686, doi: 10.1130/G33242.1.
- Taylor, G. J. (June 2004) The Multifarious Martian Mantle. Planetary Science Research Discoveries. http://www.psrd.hawaii.edu/June04/martianMantle.html.
|
|
[ About PSRD | Archive | CosmoSparks | Search | Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |
