Citation: Taylor, G. J. (July, 2019) Recipe for Making H2O in the Lunar Regolith: Implant Solar Wind Hydrogen and Heat with Micrometeorite Impacts, PSRD, http://www.psrd.hawaii.edu/July19/water-lunar-regolith.html.

|
July 12, 2019
Recipe for Making H2O in the Lunar Regolith: Implant Solar Wind Hydrogen and Heat with Micrometeorite Impacts
--- Laboratory experiments simulating space weathering on the Moon show that water can be produced by rapid heating caused by micrometeorite impacts on grains implanted with hydrogen from the Sun.
Written by G. Jeffrey Taylor
Hawai'i Institute of Geophysics and Planetology
Numerous remote sensing missions have detected hydrogen species — hydrogen (H2), hydroxyl (OH), water (H2O) — on the Moon. The missions have used a variety of ways to detect and quantify the abundance of these compounds. Data show that polar regions have lots of hydrogen and almost certainly much (maybe even most) of it is in water ice (solid H2O). One source of the water could be from solar wind implantation of hydrogen everywhere on the Moon, coupled with the reaction of the hydrogen with oxygen to make H2O on the surfaces of lunar dust grains. However, laboratory studies and theoretical calculations raise the question of efficacy of converting implanted H into H2O. New experiments by Cheng Zhu and co-workers at the University of Hawai'i reveal a pathway from H to water. Using a high-vacuum chamber to simulate conditions on the lunar surface, Zhu and colleagues implanted hydrogen (in the form of deuterium ions) into silicate dust, then used a high-powered laser to blast the dust, providing a thermal spike like that delivered by the impact of micrometeorites on the lunar surface. The experiments used mass spectrometry during the laser blasting and detailed examination of the dust using electron microscopy to show clearly that water was produced during the simulated micrometeorite bombardment. Add hydrogen to dry dust, bombard with high-velocity micron-sized projectiles, and you've got a recipe to make water in lunar regolith.
Reference:
- Zhu, C., Gillis-Davis, J. J., Crandall, P. B., Ishii, H. A., Bradley, J. P., Corley, L. M., and Kaiser, R. I. (2019) Untangling the Formation and Liberation of Water in the Lunar Regolith, Proceedings of the National Academy of Sciences, v. 116(23), p. 11165-11170, doi: 10.1073/pnas.1819600116. [abstract]
- PSRDpresents: Recipe for Making H2O in the Lunar Regolith: Implant Solar Wind Hydrogen and Heat with Micrometeorite Impacts --Short Slide Summary (with accompanying notes).
Water Ice on the Moon
The prospect of ice accumulating in the floors of polar lunar craters was first suggested in 1961 by Caltech researchers Kenneth Watson, Bruce C. Murray, and Harrison Brown. Study of lunar samples returned by the Apollo missions led to a consensus that the Moon contained little if any water, so the imaginative suggestion by Watson and coworkers fell off the lunar science radar screen. Undaunted by these results, in the late 1970s, James Arnold of the University of California, San Diego, suggested that impacting comets and water-rich asteroids could add water to the lunar surface. He argued that although most H2O molecules would be torn apart by sunlight into its constituent atoms of hydrogen and oxygen and then lost into space, some would migrate by hopping along to places where it is bitterly cold. Arnold speculated that the lunar polar regions might have areas that are permanently shadowed, hence permanently cold. The water might accumulate in these frigid shadows of the polar landscape.
Thanks to robotic orbital missions to the Moon beginning in the mid-1990s (such as Clementine, Lunar Prospector, Selene, Chang'e 1, Chandrayaan-1, Lunar Reconnaissance Orbiter (LRO), LCROSS, and LADEE) we now know much more about the lighting conditions at the North and South polar regions, have identified the locations of areas inside polar craters that are permanently shaded from direct sunlight, and have a better idea of the concentration of hydrogen and water near the surface. The hydrogen-distribution map below, derived from LRO instrument data, shows areas that potentially contain water. They are in permanent shadow and strikingly cold (mostly colder than 110 K, some places as cold as 25 K). The first direct hint of water ice was from a radar experiment done by the Clementine mission (see PSRD article: Ice on the Bone Dry Moon). The surprising lighting conditions at the poles is described in the PSRD article: The Moon's Dark, Icy Poles.
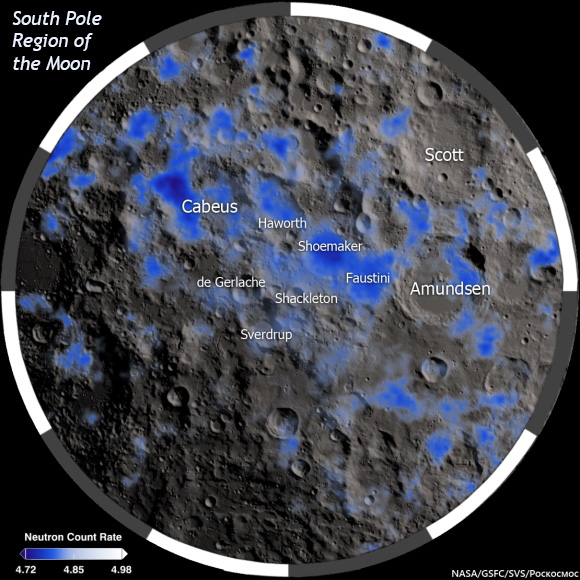 |
| A map showing permanently shadowed areas in the South Polar region of the Moon. Neutron absorption data gathered by the LEND (Lunar Exploration Neutron Detector) instrument on NASA's Lunar Reconnaissance Orbiter (LRO) shows enrichment in hydrogen in these areas (colored blue). The image makes it appear that polar regions host vast ice rinks, but in reality the areas contain relatively small amounts of water coating grains and perhaps filling voids between grains. The LCROSS mission smashed a heavy spacecraft into one of the dark regions and instruments on board LRO showed that the ejecta plume created by the impact contained 5.6 ± 2.9 weight percent H2O. |
How much water could be tied up in these lunar polar regions? The late Paul Spudis estimated that there could be between 100 million and a billion metric tons of H2O ice in the polar regions. The LCROSS impactor smashed into a permanently shadowed place near the South Pole, releasing a plume in which both gaseous water molecules and ice particles were detected by instruments on the LRO spacecraft. Analysis of the plume suggests that the cold, dark target contained 5.6 ± 2.9% H2O by weight. In contrast, an American instrument called Moon Mineralogy Mapper on the Indian Chandrayan-1 orbital mission and analyses of Apollo samples indicate that non-polar lunar regolith contains on average about 50 parts per million hydrogen (which translates to about 0.1 wt% H2O if all the hydrogen were in the form of water molecules, but that is highly unlikely). For a concise review of the amount of water that could be in the permanently shadowed areas on the Moon, read "How Much Water Is on the Moon" written in 2018 by Paul Spudis for Smithsonian Air & Space.
A billion metric tons of water is about double the amount of water in Lake Erie (located on the border between the United States and Canada). If converted back to hydrogen and oxygen, this could provide propellant for rockets operating all over the inner Solar System. That might actually happen in the future, but there is also a complicated and fascinating scientific problem to work on right now. It involves figuring out the sources of water, the varying additions of water with time, and how much is lost as it migrates to the poles. Sources could be the impact of comets (which are mostly water ice) or wet asteroids (some carbonaceous chondritic meteorites contain 20 wt% H2O, mostly residing in hydrous minerals and organic compounds), the lunar interior via volcanic eruptions early in lunar history or released by moonquakes and impacts (needs to be quantified), and hydrogen implanted into regolith grains by the solar wind converted into H2O.
Laboratory Simulation Experiments
Cheng Zhu and colleagues focused on solar-wind-implanted hydrogen and its conversion to H2O. The Moon is immersed in a stream of electrons, protons (hydrogen nuclei) and alpha particles (helium nuclei) from the Sun. The stream of particles is called the solar wind. It delivers about 300 million protons per second to each square centimeter of the surface. The Moon lacks an atmosphere, so the solar wind particles smack into the lunar surface at velocities between 250 and 600 kilometers per second (a few hundred thousand miles per hour). The fast-moving protons sputter material from grains, including previously implanted hydrogen. Most of the incoming protons bounce off, entering the flimsy lunar atmosphere (properly called the lunar exosphere), but some are implanted into solid grains. Despite their high speeds, their low masses limit their penetration depths to a few tens of nanometers, though some diffuse deeper, but only up to about a micrometer.
 |
| Photograph of the <150 micrometer size fraction of two lunar regolith (sometimes simply called soil) samples, one from the Apollo 16 site in the highlands (67511) and one from the Apollo 17 site in the maria (75061; the 7 is only partly visible in the photo). Each pile contains thousands of grains, essentially all of which have been exposed to the solar wind. |
The solar wind protons that do manage to implant themselves into a lunar dust grain do not simply come to rest as hydrogen ions. They can interact with electrons in the lunar grains to become neutral hydrogen atoms. In addition, the high energy of the penetrating protons in the solar wind break the chemical bonds between oxygen and adjacent atoms such as silicon (Si). The now partially unattached oxygen is available for hydrogen to bond with it, creating a hydroxyl (OH-) molecule. (The hydroxyl is actually part of the larger silicate structure and is attached to Si, producing SiOH.) For simplicity, all that needs to be done after manufacture of the OH radical is to add a hydrogen to it, and presto, it makes H2O. The downside is that the "presto" requires that the temperature be at least 450 K, higher than even the noontime temperature of the lunar surface in equatorial regions, which is about 400 K. (Nighttime temperature goes down to about 100 K, way too cold for the water-production process to operate.)
Cheng Zhu and colleagues decided to test the idea that micrometeorite impacts could add enough heat to make the reaction work efficiently. (For the chemists reading this, the reaction is SiOH+SiOH → SiOSi+H2O.) This required using an ultra-high-vacuum chamber, available in the W.M. Keck Research Laboratory in Astrochemistry in the Department of Chemistry at the University of Hawai‘i at Mānoa. The chamber can be pumped down to a pressure of 10 trillionth that of the Earth's atmosphere at sea level, and close to that of the essentially airless Moon. Cheng used samples of the mineral olivine, a good representative of the silicate minerals making up the dusty lunar surface. The olivine was ground to a powder smaller than 45 micrometers in diameter, which is close to the average size of dust grains in lunar regolith. The sample was pressed onto a polished silver substrate that was cooled to 10 K, at the start of the experiment. So, the set-up was a silicate mineral powder on a way cold substrate in a chamber pumped down to a pressure 10 trillion times lower than the air we breathe.
The chamber has assorted devices dangling off of it. For their experiments, Cheng Zhu and coworkers used an ion gun that simulated solar wind implantation. Specifically, they used a "quadruply differentially pumped SPECS IQE 12/38 ion gun," which PSRD thinks sounds like a defensive weapon used by crew members of the Starship Enterprise. The chamber also had a mass spectrometer attached to analyze gases given off to determine if the experiment made water molecules. To simulate micrometeorite impact and heating, the team used a high-powered laser, specifically a "SYNRAD Firestar 4.0 5 KHz carbon dioxide laser," apparently also borrowed from the Starship Enterprise. To avoid false signals from terrestrial water, which is ubiquitous, the sample was bombarded with D2+ ions. Deuterium (D) is hydrogen with a neutron added to its nucleus. It is about 6400 times lower in abundance than hydrogen in most terrestrial materials (such as ocean water), so contamination from D2O in the olivine powder or inside the chamber is not likely. During each experimental run, the samples were bombarded with an amount of deuterium equal to the amount of hydrogen a spot on the Moon receives in about 300 years.
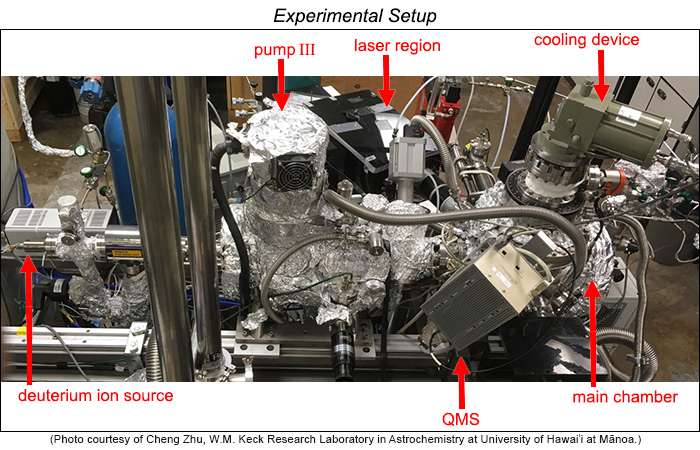 |
| Picture of the experimental setup used by Zhu and colleagues. The main chamber that holds the sample is on the right. On top of it is the apparatus that allows a sample to be cooled to a temperature as low as about 3 Kelvin (the experiment used 10 K). The deuterium ion source is on the left. The pump (labeled pump III) is the hard-working unit that lowers the pressure in the vacuum chamber. QMS is the quadrupole mass analyzer, which allows investigators to count the number of ions with specific mass-to-charge (m/z) ratio. In Zhu's experiments the ions are D2+ (m/z = 4), D2O+ (m/z = 20). The laser system generates high-energy pulses of laser light onto the sample located in the main chamber. |
Zhu and coworkers ran the experiments in two phases. In the first, they irradiated the samples with D2+ ions, but without zapping them with the laser, hence not simulating micrometeorite impact. They irradiated each sample with D2+ ions at 10 K for a simulated 300 years, then held the sample at 10 K for 120 minutes, and finally raised the temperature to 300 K. During this entire period, no D2O+ ions were detected by the mass analyzer above the background level, indicating that ion bombardment alone did not produce D2O molecules. Thus, the authors conclude, solar wind bombardment by itself is not robust enough to produce H2O in the lunar regolith.
In the second phase, Cheng Zhu tested the idea that water might be produced if energy were added by micrometeorite impacts. They followed the same procedure as in the first phase, but this time heated the sample with the laser. This temporarily raised the temperature to about 1400 K, well above the temperature thought to be needed to generate water (more than 400 K, as noted above). In this case D2+ ions were released, as measured by the mass analyzer. The flux of D2+ ions starts when the laser irradiation starts and falls when it is turned off. More importantly, a clear signal at m/z of 20 (D2O+ ions) was also produced (red curves in the figure below), beginning the moment the laser was turned on. In the two runs at 300 K, the intensity of the D2O+ signal drops when the laser is turned off. This is definitive evidence that water (in the form of D2O+ in the experiment) was made when a thermal spike was added to the sample, whether at 10 K or 300 K.
 |
| This plot shows three profiles of ion current as measured by the quadrupole mass analyzer (y-axis) versus time (x-axis) for an olivine powder held at 10 K (left profile) or 300 K (middle and right profiles). The labels Start and Stop refer to when the laser was turned on or off. Blue curves are for D2+ ions (mass ratio of 4) and red curves are for D2O+ ions (mass ratio of 20). The same graphs made for mass ratio of 20 when the laser was not used were all at the background level, indicating no water production without the laser-added heat. Cheng Zhu and colleagues conclude that these results show that H2O molecules can be produce by the combination of solar wind hydrogen implantation coupled with micrometeorite impacts. |
A Close Look at the Samples
What effect did water production and release have on the olivine powders? Is there independent evidence for water production? To answer these questions, Cheng Zhu's colleagues examined the ion-beam-exposed and laser-zapped samples using scanning and transmission electron microscopy. Specifically, they appear to have acquired some tools from the science area of the Starship Enterprise (which is located near the sensor array): They used a FEI Helios 660 dual-beam FIB (Focused Ion Beam) for scanning electron microscopy (SEM) and making ultrathin samples for study in the FEI high-base Titan G2 60-300 mono-chromated transmitted electron microscope (TEM) equipped with a Gatan Tridiem GIF (Gatan Imaging Filter). This impressive high-tech gear is located in the Advanced Electron Microscopy Center (AEMC) at the University of Hawai‘i, which is directed and managed by co-authors Hope Ishii and John Bradley.
An SEM image is shown below of an olivine grain after exposure to the ion beam and laser irradiation. The grains have a melted surface speckled with pits and covered with micron-sized grains of olivine (the powdered sample used in the experiments was all less than 45 micrometers in size, so some of it was certainly submicron in size). The pits are often accompanied by a complementary lid, flung off to the side when D2O burst out from below.
 |
| High-resolution scanning electron microscope image of the surface of an olivine grain (gray, pitted background) after ion beam exposure and laser irradiation. (The lighter aggregates are smaller olivine grains.) The round pits are places where D2O erupted from beneath, leaving behind a cavity. In two places a round lid lies adjacent to the cavity it had covered before pressure built up enough to pop it off. Image credit: Courtesy of Hope Ishii, Advanced Electron Microscopy Center, University of Hawaii. |
Electron microscopy also allowed the investigators to look at a section of an ultra-thin slice across one of the pits and perpendicular to the surface seen in the image above. The image below was taken with a scanning transmission electron microscope of a thin slice taken perpendicular to one of the pits shown above. It was sampled using a FIB (focused ion beam) device. The slice is less than 100 nanometers thick, allowing it to be transparent to electrons in the TEM. A layer of platinum (Pt) deposited by an electron beam and then one deposited by an ion beam stabilized the delicate thin slice as it was ion-milled to the desired thickness. The olivine grain was undamaged and still crystalline deeper than a depth of about 100 nanometers, but is amorphous (non-crystalline) above the undamaged region. The amorphous region contains numerous little holes, called vesicles, good evidence for the presence of a fluid (gas or liquid). Zhu and colleagues argue that the pit formed when vesicles coalesced, building enough pressure to overcome the strength of the amorphous olivine and to breach the surface, tossing a portion of the original surface aside, as seen in the previous photo. This is consistent with the formation of D2O in the sample, providing independent evidence for the synergistic roles of solar wind irradiation and micrometeorite impacts in producing water in the lunar regolith.
 |
| Cross-sectional view of a pit using transmission electron microscopy. Above the undamaged, crystalline olivine, an amorphous region contains numerous vesicles, good evidence for the presence of gas or liquid. Zhu and colleagues argue that the pit formed when vesicles coalesced and broke the surface. (Using standard procedures, the olive grain was coated with carbon (C) and platinum (i-Pt, e-Pt) prior to electron microscopy examinations. |
The experiments show clearly that H2O can be produced on the lunar surface from a combination of implantation of solar wind hydrogen and thermal spikes caused by bombardment with micrometeorites. Thus, water from the regolith could be a major source of water deposited in permanently shadowed areas in lunar polar regions. Other sources (volcanic eruptions, impact of comets or wet asteroids, the lunar interior via volcanism or mobilization by moonquakes and impacts) are also possible. Deciphering the relative importance of them will require extensive sampling of the polar ice deposits. Such sampling may be done during future missions designed to evaluate the abundance, distribution, and origin of water on the lunar surface. Today, cross-disciplinary groups of researchers are addressing key science and technology issues related to characterizing, storing, and using water and other volatiles on the Moon, such as the ICE FIVE-O team (Interdisciplinary Consortium for Evaluating Volatile Origins), led by co-author Jeffrey Gillis-Davis as one of NASA's Solar System Exploration Research Virtual Institutes (SSERVI).
- PSRDpresents: Recipe for Making H2O in the Lunar Regolith: Implant Solar Wind Hydrogen and Heat with Micrometeorite Impacts -- Short Slide Summary (with accompanying notes).
- Arnold, J. R. (1979) Ice in the Lunar Polar Regions, Journal of Geophysical Research, v. 84(B10), p. 5659-5667, doi: 10.1029/JB084iB10p05659. [abstract]
- Watson, K., Murray, B. C., and Brown, H. (1961) On the Possible Presence of Ice on the Moon, Journal of Geophysical Research, v. 66(5), p. 1598-1600. [view article]
- Watson, K., Murray, B. C., and Brown, H. (1961) The Behavior of Volatiles on the Lunar Surface, Journal of Geophysical Research, v. 66(9), p. 3033-3045. [view article]
- Zhu, C., Gillis-Davis, J. J., Crandall, P. B., Ishii, H. A., Bradley, J. P., Corley, L. M., and Kaiser, R. I. (2019) Untangling the Formation and Liberation of Water in the Lunar Regolith, Proceedings of the National Academy of Sciences, v. 116(23), p. 11165-11170, doi: 10.1073/pnas.1819600116. [abstract]
|
|
[ About PSRD | Archive | CosmoSparks | Search | Subscribe ] [ Glossary | General Resources | Comments | Top of page ] |
